ABSTRACT
Conventional cancer treatments and diagnosis, such as surgery, radiotherapy, chemotherapy, biopsy, and X-rays have been used for decades to detect cancer and to treat the patients who suffer from the malady symptoms, however the conventional cancer treatment is harmful to healthy cells, and the conventional cancer detection is not sufficiently sensitive, because it is possible only after substantial growth of cancerous cells. Novel strategies for the treatment and detection of cancer, such as Nanotechnology may greatly enhance the cure opportunities for patients and improve the quality of their life by minimizing the toxic effects of anticancer drugs. In the Nanotechnology methods, such as Sodium Chloride Nanoparticles (SCNPs), Liposomes, Magnetic Nanoparticle (MNPs), Quantum Dots (QDs), and Gold Nanoparticles (AuNPs), the nanoparticles can be designed and formulated to create therapeutic agents that targeting the cancerous cells. In this review, the details of these possi-ble detection, diagnosis and treatment methods of nanotechnology are discussed carefully.
Keywords: Cancer; Treatment; Nanotechnology; Nanoparticles; Liposome; Biopsy; Magnetic Nanoparticles; Magnetic Resonance Imaging; Quantum Dots
Abbreviations: SCNPs: Sodium Chloride Nanoparticles; MNPs: Magnetic Nanoparticle; QDs: Quantum Dots; AuNPs: Gold Nanoparticles; CNTs: Carbon Nanotubes; MRI: Magnetic Resonance Imaging; IONPs: Iron Oxide Nanoparticles; AuNPs: Colloidal Gold Nanoparticles; QDs: Quantum Dots
Introduction
The large number of cancer patients and the resulting deaths made cancer the center of attention. Ac-cording to the Center for Cancer Statistics in America in 2022, every minute four cases of cancer are diagnosed, and one patient dies [1]. Cancer is defined as a rapid random proliferation of cells. Its resistance to programmed cell death is what distinguishes it from normal cells which undergo programmed death when they malfunction [2,3]. The cancer cells may undergo metabolic changes that support their rapid growth and division [4]. The most important causes of cancer are aging, tobacco, cancer family history, alcohol, exposure to sunlight and to radiation [5]. The type of tumor, its spread and the age of the patient play the major role in conventional cancer treatment and diagnosis, such as biopsy, surgery, chemotherapy and radiotherapy [3]. An excisional biopsy could be used for conventional diagnosis and treatment, but it could not be per-formed in many types of cancers such as leukemia. In addition, the patient’s age can be a contraindication for surgery [5,6]. One of the traditional treatments for leukemia and lymphoma is chemotherapy, but some tumors do not respond to chemotherapy such as colon and pancreas cancers, in addition to its significant side effects such as hair loss, gastro-intestinal disturbance and lack of selectivity [7,8]. Skin and pancreatic cancers don’t respond well to radiotherapy, which has significant side effects, such as infertility [8]. Recently, novel methods of treatment and diagnosis of cancer have emerged, including nanotechnology that depends on the very small size of drugs (1- 100 nanometer), which helps in their penetration through different cellular barriers without causing damage to healthy cells, and consequently leading to have the desired therapeutic effect at the tumor site [9]. The aim of this work is to shed light on some recent and promising nanotechnologies that are used in the treatment and diagnosis of cancer.
Nanotechnology in Cancer Treatment
Nanotechnology has been used in medicine, especially in the targeted treatment of cancer [10,11]. The advantages consist of the easily cross of cells barriers, control size and shape, increasing efficacy and reducing toxicity of anticancer drugs [12,13]. All these features, and more, have made nanotechnology the focus of attention [13].
Sodium Chloride Nanoparticles and Cancer (SCNPs)
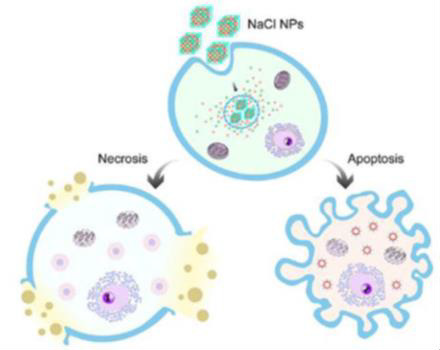
The use of salt nanoparticles is one of the most recent methods for cancer treatment that combines nano-science and immunotherapy NaCl nanoparticles were studied and found to have the ability to eliminate tumor by 66% in the 16th day of starting treatment in experimental mice and 20% of mice became completely free of tumor without causing serious side effects, such as the toxicity to major organs, hair loss or weight loss in experimental mice which lived 8 months after the treatment. The mechanism by which these particles act was causing a dramatic increase in the osmolality of the cancer cell and this leads to apoptosis and necrosis. The high selectivity of NaCl nanoparticles is because the cancer cell contains more [Na+] than the normal cell and so it is exposed to more shock than the normal cell. In addition, the cancer cells have greater affinity for nanoparticles, and the dose needed to kill the tumor is much lower than the dose that affects the normal cell (Figure 1) [13,14]. NaCl nanoparticles are exploited as a novel type of cancer treatment which is taken up by cancer cells through endocytosis and release large amounts of ions inside them. This causes a drastic increase of os-molarity, leading to cell apoptosis and necrosis. This process is highly immunogenic, stimulating an anti-cancer immunity that improves local and systemic tumor control [14].
Doxil® (DOX) and Nanotechnology
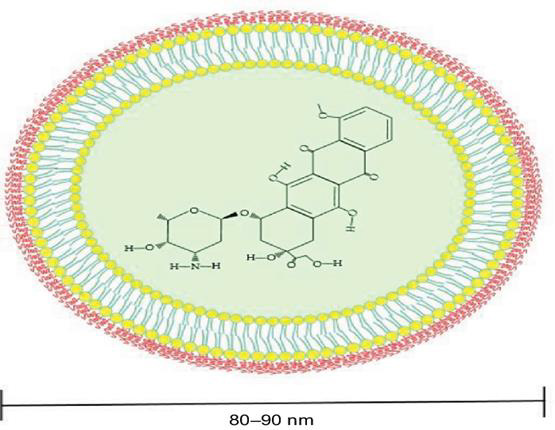
Doxil® is the first FDA-approved nano drug used to treat breast cancer, bladder cancer, Kaposi’s sarcoma, lymphoma, and acute lymphocytic leukemia [15,16]. The PEG component of Doxil® liposome causes in-creasing in blood circulation time decreasing side effects especially on heart cells [17-19], whereas Doxil® liposome is surrounded by a phospholipid bilayer and coated with methoxy polyethylene glycol. The en-capsulating Doxil® in liposomes help decreasing systemic side effects while PEGylation protects the lipo-some from recognition by the mononuclear phagocyte system and increasing its circulation time (Figure 2) [20- 22]. Doxil® Carbon Nanotubes (CNTs) can be described as graphite sheets that are rolled up into cylindrical shapes. The length of CNTs is in the form of micrometers with a diameter of about 100 nm [23]. The reasons for using carbon nanotubes are making drugs more biocompatible, fast electron transfer kinetics, ultra-light weight, chemical inertness, high tensile strength, and can be used in wide number of antibacterial and antifungal drugs, although it can act as protein carriers [24,25]. Although Doxil® liposome can be accumulated at tumors based on the enhanced permeability and retention effect, it stays around periphery of the blood vessels with limited internalization and intracellular release due to the hindrance of the PEG component [22,25,26]. Therefore, it was necessary to study other methods.
Cancer Diagnosis and Detecting
Detecting cancer at very early stages is associated with good patient prognosis as it allows immediate in-terventions to prevent cancer progression, so it is important to ensure earlier detection for effective treat-ment of cancers to reduce cancer disease spread and mortalities [27]. Among the novel strategies in cancer detecting and diagnosis is nanotechnology which has led to several promising results in the diagnosis cancer disease and detecting cancer cells, including drug delivery, gene therapy, drug carriage, biomarker mapping and targeted cancer therapy [28,29].
Magnetic Nanoparticles (MNPs)
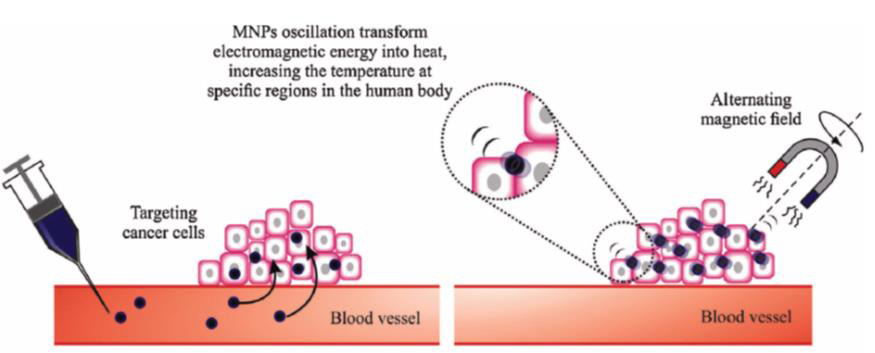
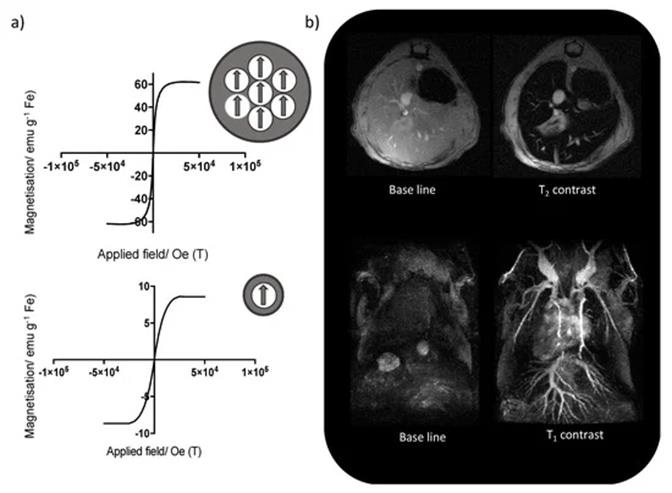
Engineered Magnetic Nanoparticles (MNPs) represent a cutting-edge tool in medicine because they can be simultaneously functionalized and guided by a magnetic field (Figure 3). Use of MNPs has advanced magnetic resonance imaging (MRI), guided drug and gene delivery, magnetic hyperthermia cancer thera-py, cancer diagnosis, tissue engineering, cell tracking and bio separation [30]. Synthesis of iron oxide magnetic nanoparticles has been achieved via physical and chemical methods [31,32]. MRI has been used as a contrast technique in cancer imaging soft tissues; nevertheless, the continuous development of magnetic nanoparticles as contrast agents has made possible the improvement of the qual-ity of the images [33]. Due to the great usefulness of the MRI technique in clinical diagnosis and the tremendous potential of iron oxide nanoparticles (IONPs), the proper functionalization of the latter (e.g., with epithelial growth factor receptor, antibodies, and short peptides sequences), as an advanced contrast agent in MRImediated molecular imaging has enabled the targeted diagnosis of various types of cancer, including breast, stomach, colon, kidney, liver, and brain cancer (Figure 4) [30,33].
Figure 4:
(a) Change in the magnetic behavior of iron oxide nanoparticles with the decrease of the core size, from superparamagnetic (top) to paramagnetic (bottom),
(b) Mouse liver T2-weighted MRI using iron oxide nanoparticles with bigger core size (top), and T1-weighted MR angiography using iron oxide nanoparticles with smaller core sizes (bottom) [30].
Consequently, a series of promising applications have emerged in these recent years, for example, an at-tractive study described the design of a short peptide and ligand library to functionalize IONPs which were able to choose the ligand that provided IONPs with the most advantageous features for in vivo ap-plications and obtaining a considerable enhancement in contrast between the liver tumor and the healthy liver tissue in comparison with a commercial MRI contrast agent [33]. In another interesting study, researchers developed fine-tuned IONPs coated with polyethylene glycol and useful in MRI cancer imaging. In vitro characterization, the results revealed high biocompatibility and relativity values greater than the commercial alternative Ferumoxytol®. The in vivo characterization with breast cancer mouse models showed the capability of these PEG-coated IONPs to be totally preserved at the tumor site for up to 24 h, thus indicating their strong potential as MRI contrast agents for real-time long-lasting monitoring of tumor progression [30,33].
Quantum Dots (QDs)
Quantum Dots (QDs) are semiconductor nanocrystals that emit fluorescence on excitation with a light source. They have excellent optical properties, including high brightness, resistance to photobleaching and tunable wavelength. Their unique optical properties, such as high brightness, long-term stability, simultaneous detection of multiple signals and tunable emission spectra, make them appealing as potential diagnostic and therapeutic systems [34,35]. The lack of ability to penetrate objects limits the use of visible spectral imaging, quantum dots that emit fluorescence in the near-infrared spectrum (700-1000 nanometers) have been designed to overcome this problem, making imaging colorectal cancer, liver cancer, pancreatic cancer, and lymphoma [36,37]. A second near-infrared (NIR) window (900-1700 nm) with higher tissue penetration depth, higher spatial and temporal resolution has also been developed to aid cancer imaging. Also, the development of silver rich quantum dots containing a sulfur source has been reported to allow visualization of better spatial resolution images over a wide infrared range. Despite all the advantages, the applications of these nanoparticles are limited for the use in human system, and this is mainly because of the toxicity associated with heavy metals [38,39].
Colloidal Gold Nanoparticles (AuNPs)
Colloidal gold nanoparticles have been prepared from HAuCl4 using aqueous solution, X-ray irradiation, and chemical reduction method. Gold nanorods were synthesized according to the seedmediated growth method. The colloidal gold nanoparticles were characterized by using transmission electron microscopy, X-ray diffraction, and UV-VIS absorption spectroscopy [40]. The literature is usually associated with controlled methods of synthesis, allowing for the acquisition of AuNPs with defined shapes and sizes (Figure 5) [41]. Gold nanoparticle is a good contrast agent because of its small size, good biocompatibility, and high atomic number. The research showed that AuNPs work by both active and passive ways to target cells. The principle of passive targeting is governed by gathering the gold nanoparticles to enhance imaging because of the permeability tension effect in tumor tissues. Mixing AuNPs with liver cancer cells and using X-ray imaging showed that the clusters of liver cancer cells in the gold nanocomposite group were significantly stronger than those in the liver cancer cells alone. These findings have important implications for early diagnosis allowing tumors as small as a few millimeters in diameter to be detected in the body [40,41].
Conclusion
Many researches have conducted to investigate nanotechnology and introduced its significant role in treating and diagnosing cancer, and that because nanotechnology has a remarkable fingerprint in the medical field and it is becoming famous due to its superiority over other technologies. The efficiency of nanotechnology in the treatment and diagnosis of cancer is proven due to the ability of controlling the particles size and shape, its selectivity towards cancer cells, the improvement of the bio-compatibility and nanoparticles do not make toxicity to the normal cells as conventional cancer treatments.
References
- (2021) American Cancer Society. Cancer statistic center.
- Nall R, MSN, CRNA (2020) Cancer: Overview, causes, treatments, and types.
- (2017) Types of Cancer Treatment.
- Dattani R Farouk R (2010) Cancer Treatment I: Surgery. In Principles of Surgery Vivas for the MRCS Cambridge University Press, p. 50-91.
- (2011) What is cancer surgery Approved by the Cancer.Net Editorial Board. Cancer.
- Alexander EJ, Cleator S (2010) Principles of chemotherapy. In: O Aziz, S Purkayastha, P Paraskeva (Eds.)., Cambridge: Cambridge University Press, pp. 575-579.
- National help service (NHS).
- Side effects of radiotherapy.
- Gmeiner WH, Ghosh S (2015) Nanotechnology for cancer treatment. Nanotechnol Rev 3(2): 111-122.
- Chen H, Gu Z, An H, Chen C, Chen J, et al. (2018) Precise nanomedicine for intelligent therapy of cancer. Science China Chemistry 61(12): 1503-1552.
- Lamberti M, Zappavigna S, Sannolo N, Caraglia M (2014) Advantages and risks of nanotechnologies in cancer patients and occupationally exposed workers. Journal of Expert opinion on drug delivery 11(7): 1087-1101.
- Ruoslahti E, Bhatia SN, Sailor MJ (2010) Targeting of drugs and nanoparticles to tumors. The Journal of Cell Biology 188(6): 759-768.
- Alshahrani A (2016) The advantages of nanotechnology in medical field. International Journal of Innovative Research in Electrical Electronics Instrumentation and Control Engineering 4(4): 1-4.
- Jiang W, Yin L, Chen H, Paschall AV, Zhang L, et al. (2019) NaCl nanoparticles as a cancer therapeutic. Ad vanced Materials Deerfield Beach Fla 31(46): 1904058.
- Barenholz YC (2012) DoxilⓇ the first FDA approved nano drug lessons learned. J Controll Release 160(2): 117-134.
- Wibroe PP, Ahmadvand D, Oghabian MA, Yaghmur A, Moghimi SM (2016) An integrated assessment of morphology size and complement activation of the PEGylated liposomal doxorubicin products DoxilⓇ CaelyxⓇ Doxorubicin, and Sina Doxosome. J Controll Release 221: 1-8.
- Webber MJ, Appel EA, Vinciguerra B, Cortinas AB, Thapa LS, et al. (2016) Supramolecular PEGylation of biopharmaceuticals. Proc Natl Acad Sci USA 113(50): 14189-14194.
- Wang H, Hu Y, Wang Y, Lu J, Lu H (2021) DoxorubicinⓇ PEPylated interferon-polydisulfides: a multi-responsive nano-particle for enhanced chemo-protein combination therapy. Giant 5(7): 100040.
- Yesenia LF, Tanaya R, Ait Oudhia S (2018) Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer- Targets and Therapy 10: 131-141.
- Chen J, Ding J, Wang Y, Cheng J, Ji S, et al. (2017) Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv Mater 29(32): 1701170.
- Jiang, N, Shen T, Ci Z, Tang Z, Gu G, et al. (2019) Combretastatin A4 nanodrug induced MMP9 amplification boosts tumor selective release of doxorubicin drug. Adv Mater 31(44): 1904278.
- L Zhang, Abdullah R, Hu X, Bai H, Fan H, et al. (2019) Engineering of bioinspired, size-controllable, self-degradable cancer-targeting DNA nanoflowers via the incorporation of an artificial sand-wich base. J Am Chem Soc 141(10): 4282-4290.
- He H, Pham Huy LA, Dramou P, Xiao D, Zuo P, et al. (2013) Carbon nanotubes applications in pharmacy and medicine. BioMed Research International 2013: 578290.
- Smart SK, Cassady AI, Lu GQ, Martin DJ (2006) The biocompatibility of carbon nanotubes. Carbon 44(6): 1034-1047.
- Zhang W, Zhai Z, Huang S, Mao Z, Zhang Y, et al. (2020) Morphological and constituent viral-mimicking self-assembled nanoparticles promote cellular uptake and improve cancer therapeutic efficiency in vivo. Giant 3: 100026.
- Fan Y, Leunig M, Shi KH, Berk DA, Papahadjopoulos D, et al. (1994) Mirovascular permeability and interstitial pene-tration of sterically stabilized (Stealth) liposomes in a human tumor xenograft. Cancer Res 54 (13): 3352-3356.
- Sun Q, Zhou Z, Qiu N, Shen Y (2017) Rational design of cancer nanomedicine nanoproperty integration and synchronization. Adv. Mater 29(14): 1606628.
- Zhu M, Nie G, Meng H, Xia T, Nel A, et al. (2013) Physicochemical properties determine nanomaterial cellular uptake transport and fate. Acc Chem Res 46(3): 622-631.
- Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, et al. (2012) Overcoming limitations in nanoparticle drug delivery: triggered, intravas cular release to improve drug penetration into tumors. Cancer Res 72(21): 5566-557.
- Fernández BI, Munoz HM, Ruiz Cabello J, Herranz F, Pellico J (2020) Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 8(4): 28.
- Adewunmi AA, Kamal MS, Solling TI (2021) Application of magnetic nanoparticles in demulsification: A review on synthesis, performance, recyclability, and challenges. Journal of Petroleum Science & Engineering 196: 107680.
- Lima Tenório MK, Pineda EAG, Ahmad NM, Fessi H Elaissari A (2015) Magnetic nanoparticles: In vivo cancer diagnosis and therapy. IJ Ph 493(1-2): 313-327.
- Liu D, Li J, Wang C, An L, Lin J, et al. (2021) Ultrasmall Fe@ Fe3O4 nanoparticles as T1-T2 dual-mode MRI contrast agents for targeted tumor imaging. Nanomedicine 32: 102335.
- Luo G, Long J, Zhang B, Liu C, Ji S, et al. (2012) Quantum dots in cancer therapy. Expert Opinion on Drug Delivery 9(1): 47-58.
- Pisanic TR, Zhang Y, Wang TH (2014) Quantum dots in diagnostics and detection: principles and paradigms. The Analyst 139(12): 2968-2981.
- Aswathy RG, Yoshida Y, Maekawa T, Kumar DS (2010) Near infrared quantum dots for deep tissue imaging. Analytical and Bioanalytical Chemistry 397(4): 1417-1435.
- Zhao P, Xu Q, Tao J, Jin Z, Pan Y, et al. (2018) Near infrared quantum dots in biomedical applications: current status and future perspective. Near infrared quantum dots in biomedical applications. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology 10(3): 1483.
- Gil HM, Price TW, Chelani K, Bouillard JSG, Calaminus SDJ, et al. (2021) NIR quantum dots in biomedical imaging and their future. IScience 24(3): 102189.
- Jin C, Wang K, Oppong Gyebi A, Hu J (2020) Application of nanotechnology in cancer diagnosis and therapy A mini-review. International Journal of Medical Sciences 17(18): 2964-2973.
- Krzysztof S, Michał G, Barbara KM (2019) Gold Nanoparticles in Cancer Treatment. Mol Pharm 16(1): 1-23.
- Piyushkumar KS, Anjali R, Avinash KS, Dillip KD, Nirmal VS, et al. (2020) A Combined Approach of Gold Nanoparticles with Cannabinoids for the Treatment of Cancer -A Review.

 Review Article
Review Article