ABSTRACT
The incidence of de novo hepatitis B virus (HBV) infection (DNHB) among patients receiving hepatitis B core antibody positive (HBcAb +ve) grafts is unacceptably high without prophylaxis (38%-100%). Therefore, it is of utmost importance to carry out effective prophylactic strategies. Two HBV naïve pediatric recipients in our center received HBcAb +ve liver grafts from living donors. One was a case of familial hypercholesterolemia and the other suffered from recurrent hepatoblastoma. Our prophylactic regimen in management of those recipients included pre-operative HBV vaccination targeting a hepatitis B surface antibody (HBsAb) titer >1000 IU/L and both intra and post-operative hepatitis B immunoglobulin (HBIg) supplementation targeting HBsAb titer of > 1000 IU/L in the first week then maintaining it above 200 IU/L in the following period. This was combined with NUCs in the first post-LT year. Both were stopped after the first post-LT year switching to HBV vaccine alone thereafter. Strict monitoring of HBsAb titer and HBV viral markers was undertaken. Untill the last visit of both patients (56- and 18-months post-LT), all their HBV markers were negative and their HBsAb titer was maintained at 1000 IU/L. We conclude that HBcAb positive grafts can be used safely in pediatric LT if an adequate prophylactic regimen is followed.
Keywords: De Novo Hepatitis B Virus Infection; Familial Hypercholesterolemia; Hepatoblastoma; Liver Transplantation; Prophylaxis
Abbreviations: AST: Aspartate Transaminase; ALT: Alanine Transaminase; BMI: Body Mass Index; cm: Centimeter; CMV: Cytomegalovirus; CT: Computed Tomography; EBV: Ebestien Bar Virus; ECHO: Electrocardiography; Hb: Hemoglobin; HBcAb: Hepatitis B Core Antibody; HBV: Hepatitis B Virus; HBsAb: Hepatitis B Surface Antibody; HCV-Ab: Hepatitis C Virus-Antibody; Ig: Immunoglobulin; Kg: Kilogram; LT: liver Transplantation; PCR: Polymerase Chain Reaction; PET: Positron Emission Tomography; TLC: Total Leucocyte Count
Introduction
Liver transplantation (LT) is the only cure for end-stage pediatric liver disease. In spite of the fact that good long-term outcome has been achieved in pediatric recipients, many questions still remain unanswered. One of the unsolved issues is the long-term prevention and management of de novo hepatitis B virus (HBV) infection (DNHB) [1]. The use of hepatitis B core antibody positive (HBcAb +ve) liver grafts is a promising strategy to expand the donor pool, however, the potential risk of DNHB is one of the major concerns of using this type of liver grafts. So far, the experience of using HBcAb +ve grafts in pediatric LT is limited [2]. Several different approaches for DNHB prophylaxis have been used in recipients of HBcAb +ve livers according to the preference and experience of each institution. These include nucleos(t)ide analogues (NUCs), hepatitis B immunoglobulin (HBIg) or a combination of both [3] and HBV vaccination [4]. We describe our experience in the management of 2 pediatric HBV naïve recipients of HBcAb +ve liver grafts from living donors, who are following at Dr. Yassin Abdel Ghaffar Charity Center for Liver Disease and Research in Egypt.
Case 1
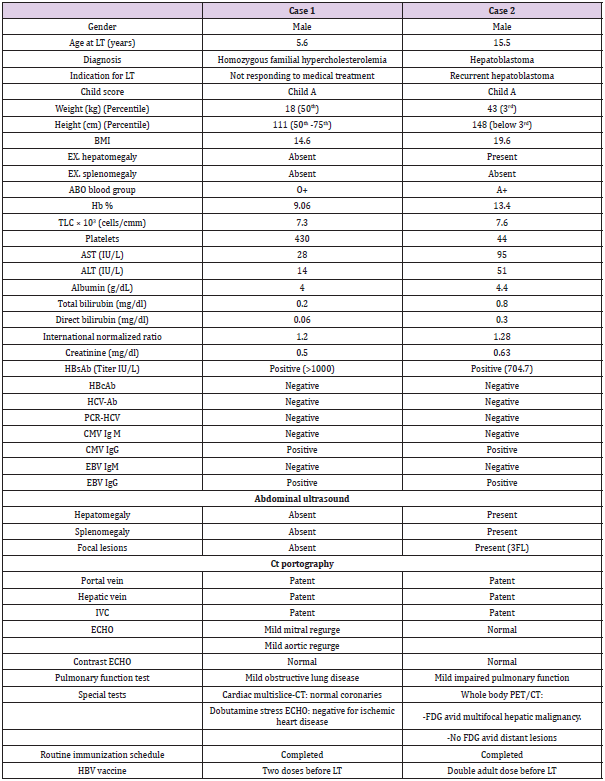
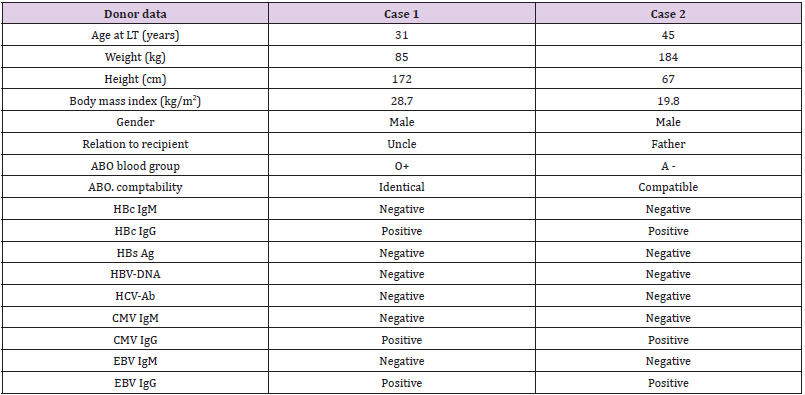
A male child, HH, who was referred to us at the age of 5.6 years for LT. He was diagnosed with homozygous familial hypercholesterolemia. His cholesterol level was persistently above 700 mg% in spite of medical treatment. His sister died at age of 6 years with the same condition. Both of his parents had hypercholesterolemia so they were not suitable for graft donation. The only available donor was his uncle, who during LT work-up was found to be HBcAb +ve but HBsAg –ve, HBsAb –ve and HBV DNA –ve. He was otherwise completely suitable for donation. The preoperative work up and donor data are presented in Tables 1 & 2. HH underwent LT on 16.6.2016. Our prophylactic regimen against DNHB involved preoperative, intraoperative, and postoperative prophylactic work up.
Table 1: Pre-operative work up of both recipients.
Note: AST: Aspartate Transaminase; ALT: Alanine Transaminase BMI: Body Mass Index; cm: centimeter; CMV: Cytomegalovirus; CT: Computed Tomography; EBV: Ebestien Bar Virus; ECHO: Electrocardiography; Hb: Hemoglobin; HBcAb: Hepatitis B Core Antibody; HBV: Hepatitis B virus, HBsAb: Hepatitis B Surface Antibody; HCV-Ab; Hepatitis C Virus-Antibody; Ig: Immunoglobulin; Kg: Kilogram; LT: liver Transplantation; PCR: Polymerase Chain Reaction; PET: Positron Emission Tomography; TLC: Total Leucocyte Count
Pre-Operative
HH had received HBV vaccine in infancy as part of the national vaccination program. His HBsAb titer before LT was zero. Two booster doses of the vaccine were given aiming at HBsAb titer of 1000 IU/L. The patient reached the target titer before LT
Intraoperative
50 IU/kg HBIg through intramuscular (IM) route were given during a hepatic phase.
Post-Operative
In the first week: HBIg was given daily for 4 days aiming at keeping the HBsAb titer above 1000 IU/L. Lamivudine at dose of 3 mg/kg/day was started on day 1 post-LT. In the first year postoperative: both Lamivudine and HBIg were used. HBsAb titer was monitored every 2 weeks in the first 3 months then monthly thereafter until the end of the first year post-LT aiming to maintain HBsAb level above 200 IU/L. A dose of 200 IU HBIg was given if the HBsAb level fell below 200 IU/L. Five more such doses of HBIg were needed in the first year post-LT to maintain the target HBsAb titer. HBcAb, HBV DNA and HBsAg were assessed every 2 to 3 months so long liver enzymes were normal but at any time they increased. In the second year post-LT we stopped both lamivudine and HBIg and gave only HBV vaccine. A double dose vaccine was given only once. Since then and up till now he needed no more doses as his HBsAb titer has been always maintained above 1000 IU/L. Follow up for HBsAb titer, HBsAg, HBcAb and HBV DNA is done every 3 months.
Immunosuppressive Regimen
HH received methylprednisolone 10 mg/kg intra-operatively then changing to 2 mg/kg/day oral prednisone and tapering it to complete withdrawal after 3 months. Maintenance immunosuppression was with tacrolimus and mycophenolate mofetil. HH cholesterol level had dropped markedly after LT but it did not reach the normal level. So he was put on statins. Since then his cholesterol was normal most of time. On his last visit on 21.2.2021 all his HBV markers were negative. His HBsAb titer was > 1000 IU/L.
Case 2
Male patient, MA, 15.5 years old, had recurrent hepatoblastoma and was referred to our center for LT. He was diagnosed at the age of 12 years to have hepatoblastoma. Left hepatectomy was done and he received chemotherapy. At the age of 14 years, tumor recurrence occurred, and MA had undergone resection for segment V plus cholecystectomy and received chemotherapy. One year later, at age of 15 years, tumor recurrence occurred again, and he received 2 doses of chemotherapy. Then he was referred for salvage LT.The only available donor was his father who was discovered during donor pre-operative work up to be HBcAb+ve but negative for HBsAg, HBsAb and HBV DNA. With the lack of another suitable donor and the need for urgent LT because of the bad condition of the recipient and the aggressiveness and frequent recurrences of the tumor, we had to accept the father as a donor. Pre-operative assessment and donor data are summarized in Tables 1 & 2. The patient underwent LT on 15.1.2018. A strict prophylactic regimen was followed. Pre-operative: MA had also received HBV vaccine in infancy as part of the national vaccination program but the HBsAb titer was found to be <2 IU/L. After double adult dose HBV vaccine, the HBsAb reached 704.7 IU/L. With his immunosuppressed state and the urgency of LT in this case, we couldn’t wait till the target pre-operative HBsAb titer (1000 IU/L) was reached.
Intraoperative
The recipient was injected with 50 IU/kg HBIg through IM route during the anhepatic phase.
First Post-Operative Week
MA had received HBIg daily for 4 days aiming at keeping the HBsAb titer above 1000 IU/L. Oral Entecavir at dose of 0.015 mg/ kg/day was started on day 1 post-LT.
In the First Postoperative Year
Both Entecavir and HBIg were used. Monitoring of HBsAb titer and the other HBV markers was as in case 1. A dose of 540 IU HBIg was given if the HBsAb level fell below 200 IU/L. Seven more doses of HBIg were required in the first year post-LT to maintain the target HBsAb titer. According to the decision of the oncologists he received 2 cycles of chemotherapy 1.5 months after LT.
On the Second Year Post-LT
Entecavir and HBIg were discontinued, and the patient received double adult HBV vaccine dose only once. He was lost to follow up 1.5 years post-LT.
Immunosuppressive Regimen
MA received methylprednisolone 10 mg/kg intraoperative, then 2 mg/kg/day prednisolone tapering it to complete withdrawal after 1 year. Maintenance immunosuppression was with tacrolimus monotherapy. One-year post-LT a recurrent hepatic focal lesion and bilateral pulmonary nodules appeared and he started palliative treatment. On his last visit on 13.1.2020 all his HBV markers were negative. His HBsAb titer was >1000 IU/L. Sadly, he passed away 2 years after LT.
Discussion
The reported incidence of DNHB infection among patients receiving HBcAb +ve grafts is unacceptably high without prophylaxis (38%-100%) as such grafts carry a high risk of occult HBV infection (defined as detectable intrahepatic HBV DNA in a HBsAg –ve person) [5]. Therefore, it is of utmost importance to carry out effective prophylactic strategies to prevent DNHB infection in children receiving HBcAb +ve grafts. Living related LT is an elective surgery, where the recipient, donor and surgical team have the chance for good preparation. Yet, it is not always easy to get a healthy suitable donor. We were faced by this problem in 2 of our pediatric patients, where the only available donors were healthy but were HBcAb +ve. One recipient was a case of homozygous familial hypercholesterolemia, a dominant trait carried by both parents and the other suffered from recurrent hepatoblastoma where intervention at the proper time was imperative.
On screening for hepatitis B virus infection in Egyptian blood donors negative for HBsAg, the prevalence of isolated HBcAb in tested samples was 13.3%. The overall prevalence of HBV DNA in healthy blood donors among isolated anti-HBc-positive individuals was 10% [6]. Our prophylactic regimen in management of recipients of HBcAb +ve grafts was similar to a great extent to that adopted by Tianjin First Central Hospital in China [7] but ours included preoperative booster doses of HBV vaccine targeting HBsAb titer above 1000 IU/L. Previous studies in both adult and pediatric HBcAb +ve liver graft recipients indicated that a preoperative HBsAb titer ≥1000 IU/L was effective in protecting recipients from HBV infection [8]. Lin, et al. reported 15.4% incidence of DNHB in patients whose HBsAb titers were between 100 and 1000 IU/L. No DNHB was seen in patients who’s anti‐HBsAb titers were kept above 1000 IU/L in that study [9]. This high titer would presumably neutralize the potential viral antigen coming from the HBcAb +ve grafts [10,11]. The high HBsAb titer that was attained preoperatively made it possible to use a lower intraoperative HBIg dose (50 IU/kg) than the one used in other studies [12,7]. In addition, a study in children indicated that the level of pre-LT HBsAb titer was associated with the response to post-LT booster vaccine [13].
Case no.1 successfully reached the pre-operative target titer of HBsAb, while case no.2 didn’t. As he had severe primary disease LT had to be performed although the target HBsAb titer was not reached. Our preoperative regimen provided the initial protection against DNHB infection, whereas the postoperative regimen provided a continuous protection as well as a salvage method for patients who did not meet the preoperative criterion. On the first year both children received a combination of HBIg and NUCs for one year aiming to maintain the HBsAb titer above 200 IU/L as postoperative HBsAb titer of <200 IU/L may increase the risk of DNHB [14]. The need to administer HBIg on long term is highly expensive, also the long-term use of lamivudine may raise the concern of mutant strains [5,15], so we shifted to active immunization with HBV vaccine starting from the second year post-LT. Because HBV vaccine response rates are lower in the early post-LT period, we used HBIg for maintenance of sufficient anti‐HBsAb levels during the first post-transplant year, when the level of immunosuppression is highest.
In the study of Park, et al. the median follow-up duration after vaccination was 26.5 months, and a median of 2.03 doses of vaccine per year was required for the maintenance of anti-HBs titers greater than at least 100 IU/l (4) while our patients were followed for 56 months in case 1 and 18 months in case 2, and only one double dose vaccine was required by each during the whole follow up period. Yoshizawa, et al. [16] reported that in the pediatric population post-LT double dose vaccination was effective in more than 80% of cases and a high titer of 1000 IU/L could be achieved even if pre-LT vaccination was not performed [16]. It is of not worthy to mention that in the first post-LT year, case 2 compared to case1 had a more rapid decay in HBsAb titer (1 month vs 3 months) and also needed more doses (7 doses) with higher HBsAb levels to maintain the target titer >200 IU/L. this may have been related to his immunosuppressed state specially that he received 2 chemotherapy cycles starting 1.5 months post-LT. Children treated for cancer are immunosuppressed during treatment and for a variable period after completion of chemotherapy. There is a reduction of vaccine-antigen specific antibody concentration for some time after the cessation of chemotherapy as chemotherapy is toxic for lymphocytes [17,18].
Early steroid withdrawal and low maintenance level of tacrolimus makes effective DNHB prophylaxis possible [19]. Steroid was considered to increase the risk of post-LT HBV infection as it may stimulate the glucocorticoid responsive element present in the viral genome thus further up regulating HBV gene expression [20]. Lifelong prophylaxis, continuous monitoring, and compliance are imperative for success of the prophylactic regimen. During the follow up period, HH and MA did not develop DNHB. Lifelong monitoring of HBV serologic status is necessary in these patients because DNHB has been known to occur late in the post-LT period [21]. probably due to development of escape mutants where there is loss of immunoreactivity against the variant despite presence of HBsAb.
Conclusion
Pre-operative vaccination with HBV vaccine targeting HBsAb titer >1000 IU/L followed by intra and post-operative HBIg supplementation combined with NUCs in the 1st post-LT year and switching to HBV vaccine thereafter was an effective prophylactic regimen against DNHB infection in our pediatric recipients receiving HBcAb +ve grafts. HBcAb +ve grafts can safely be used in pediatric LT if an adequate prophylactic regimen is followed.
Conflict of Interest
Not declared.
Source of Funding
Non-Fundable Study.
References
- Dong C, Song Z, Chen J, Ma N, Meng X, et al. (2020) Risk factors of de novo hepatitis B virus infection in pediatric hepatitis B core antibody positive liver graft recipients under prophylactic therapy. J Gastroenterol Hepatol 35(5): 827-832.
- Song Z, Dong C, Meng X, Sun C, Wang K, et al. (2021) Prophylactic Strategy Against De Novo Hepatitis B Virus Infection for Pediatric Recipients Who Receive Hepatitis B Core Antibody-Positive Liver Grafts. Liver Transpl 27(1): 96-105.
- Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, et al. (2015) Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant 15(5): 1162-1172.
- Park JB, Kwon CH, Lee KW, Choi GS, Kim DJ, et al. (2008) Hepatitis B virus vaccine switch program for prevention of de novo hepatitis B virus infection in pediatric patients. Transpl Int 21(4): 346-352.
- Lin CC, Yong CC, Chen CL (2015) Active vaccination to prevent de novo hepatitis B virus infection in liver transplantation. World J Gastroenterol 21(39): 11112-11117.
- El-Sherif AM, Abou-Shady MA, Al-Hiatmy MA, Al-Bahrawy AM, Motawea EA (2007) Screening for hepatitis B virus infection in Egyptian blood donors negative for hepatitis B surface antigen. Hepatol Int 1(4): 469-470.
- Dong C, Gao W, Ma N, Sun C, Zheng WP, et al. (2017) Risks and treatment strategies for de novo hepatitis B virus infection from anti-HBc-positive donors in pediatric living donor liver transplantation. Pediatr Transplant 21(2): e12854.
- Wang SH, Loh PY, Lin TL, Lin LM, Li WF, et al. (2017) Active immunization for prevention of De novo hepatitis B virus infection after adult living donor liver transplantation with a hepatitis B core antigen-positive graft. Liver Transpl 23(10): 1266-1272.
- Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, et al. (2007) Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant 7: 195-200.
- Shi B, Wu Y, Wang C, Li X, Yu F, et al. (2019) Evaluation of antiviral passive active immunization (“sandwich”) therapeutic strategy for functional cure of chronic hepatitis B in mice. E Biomedicine 49: 247-257.
- Koivisto K, Puhakka L, Lappalainen M, Blomqvist S, Saxén H, et al. (2017) Immunity against vaccine-preventable diseases in Finnish pediatric healthcare workers in 2015. Vaccine 35(12): 1608-1614.
- Yamashiki N, Yoshizawa A, Ueda Y, Kaido T, Okajima H, et al. (2018) The use of hepatitis B immunoglobulin with or without hepatitis B vaccine to prevent de novo hepatitis B in pediatric recipients of anti-HBc-positive livers. Pediatr Transplant 22(5): e13227.
- Kwon CH, Suh KS, Yi NJ, Chang SH, Cho YB, et al. (2006) Long-term protection against hepatitis B in pediatric liver recipients can be achieved effectively with vaccination after transplantation. Pediatr Transplant 10(4): 479-486.
- Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, et al. (2009) High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr 48(2): 203-208.
- Sánchez Fueyo A, Rimola A, Grande L, Costa J, Mas A, et al. (2000) Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: A new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. Hepatology 31(2): 496-501.
- Yoshizawa A, Yamashiki N, Ueda Y, Kaido T, Okajima H, et al. (2016) Long-term efficacy of hepatitis B vaccination as post-transplant prophylaxis in hepatitis B surface antigen (HBsAg) positive recipients and HBsAg negative recipients of anti-hepatitis B core positive grafts. Hepatol Res 46(6): 541‐551.
- Shams Shahemabadi A, Salehi F, Hashemi A, Vakili M, Zare F, et al. (2012) Assessment of antibody titers and immunity to Hepatitis B in children receiving chemotherapy. Iran J Ped Hematol Oncol 2(4): 133-139.
- Shin HJ, Lee ES, Han SB, Lee JW, Chung NG, et al. (2019) Serological Changes against Hepatitis B Surface Antigen in Children and Adolescents Receiving Chemotherapy for Acute Leukemia. Mediterr J Hematol Infect Dis 11(4): e2019052.
- Lee S, Kim JM, Choi GS, Park JB, Kwon CH, et al. (2016) De novo hepatitis b prophylaxis with hepatitis B virus vaccine and hepatitis B immunoglobulin in pediatric recipients of core antibody-positive livers. Liver Transpl 22(2): 247-251.
- Raimondo G, Filomia R, Maimone S (2014) Therapy of Occult Hepatitis B Virus Infection and Prevention of Reactivation. Intervirology 57(3): 189-195.
- Bohorquez HE, Cohen AJ, Girgrah N, Bruce DS, Carmody IC, et al. (2013) Liver transplantation in hepatitis B core‐negative recipients using livers from hepatitis B core‐positive donors: a 13‐year experience. Liver Transpl 19(6): 611‐618.

 Case Report
Case Report