ABSTRACT
The inflammatory nature of Parkinson’s disease (PD) in the central nervous system contains inflammatory structures such as alpha-synuclein, interleukins and several other modulators. In view of this characteristic, it is observed the possibility of using anti-inflammatory drugs to combat the injury profile of structures related to the pathophysiology of the disease. This study aims to do a review of the literature on preclinical studies addressing the efficacy of anti-inflammatory drugs in PD. For this study has performed a systematic review elaborated according to the Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P)5. Pubmed, LILACS, EMBASE, MEDLINE and Web of Science databases were searched in the period 2000-2019, without language restriction, containing clinical or pre-clinical studies based on the association of keywords: Parkinson’s; corticosteroid; anti-inflammatory; neuroprotection. The articles were independently selected by two reviewers in three stages: screening by title, by abstract and by full reading of the article. All articles were investigated for their methodological quality using the ACROBAT-NRSI scale (“A Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies”) 4. We have found Twenty-one articles that were analyzed and separated between two groups according to the drug used for preclinical study, anti-parkinsonian drugs of anti-inflammatory nature and drugs properly anti-inflammatory drugs (IINES and corticosteroids). Improvement in motor function, decreased movement patriotization, increased levels of striatal dopamine, decreased interleukins and blockage of inflammatory pathways, such as those participating in MPP+ and COX-2, as well as increased and/or decreased loss of neurons armed with tyrosine hydroxylase (TH) enzyme, an important marker of neuroprotection, were identified. Thus the analyzed articles demonstrated an effective anti-flame response and effective neuroprotection in the two groups of drugs, with a therapeutic alternative and promising line of study in PD.
Keywords: Parkinson’s; Anti-Inflammatory; Neuroprotection
Abbreviations: PD: Parkinson’s Disease; AD: Dopamine Neurons; TH: Tyrosine Hydroxylase
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative
disease characterized by the loss of dopamine neurons (AD) in
the substance nigra pars compacta (CNS) and accumulation of
insoluble cytoplasmic protein inclusions called Lewy and Lewy
neurites bodies [1]. The precise mechanism underlying the
pathogenesis of PD is not yet fully understood. The accumulation
of evidence suggests that soluble α-synuclein aggregates, known as
oligomers, play a significant role in PD where the neurodegenerative
process culminates in impairing several subcellular functions
[1]. Thus, clinically, PD presents as muscle stiffness, tremor at
rest, bradykinesia (abnormal slowness of voluntary movements), postural instability; some patients also have symptoms related to
psychiatric and cognitive disorders. In this context, intraneuronal
accumulation and aggregation of alpha-synuclein can start from
several sites such as the intestinal tract, where this altered protein
(alpha-synuclein) can be transported through the enteric route to
the CNS through the parasympathetic pathway [2]. In addition to
this hypothesis, there is genetic influence in the functional roles
of genes identified as monogenic forms of PD. Mutations in SNCA,
LRRK2 and VPS35 genes have been highly penetrating and cause
autosomal dominant forms of PD [1]. Thus, showing the existence
of multifactorial processes to support the underlying cause of this
aberrant protein accumulation. Therefore, what most of these
studies show is that when alpha-synuclein is lodged in the CNS
itself, it is directly linked to damage triggered by the activation
of microglia, which, by releasing inflammatory factors, causes an
oxidative burst affecting neuronal cells leading to death [3].
Thus, since there is a pattern of inflammatory characteristics
after the beginning of the accumulation of these proteins, this tangle
of interleukins, TNF-α, TNF-γ, CCL2, ROS and NO may increase such
accumulation and aggregation already in force, thus determining
an even more cumulative and oxidative neurodegenerative picture,
exponentially affecting the patient’s condition, becoming a real
“Parkinson’s snowball”. Thus, this hypothesis suggests a clinical
applicability of treatment with anti-parkinsonian drugs of antiinflammatory
nature and drugs properly anti-inflammatory drugs
(IANES and corticosteroids), where the anti-inflammatory action
may provide a therapeutic resource for patients with the purpose
of promoting a decrease in levels of dopaminergic cell lesions and
lowering of alpha-synuclein accumulation. This study, therefore,
aims to correlate the use of these two types of drugs with antiinflammatory
attributes to the treatment of PD, observing whether
there is an anti-inflammatory or neuroprotective response (via
dopaminergic markers) and which group of drugs is better than the
other.
Methodology
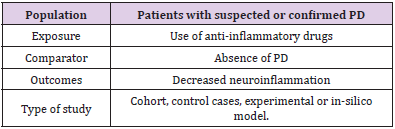
This study consisted of a systematic review prepared according to the Preferred reporting items for systematic review and metaanalysis protocols (PRISMA-P). The eligibility criteria defined for the inclusion of an article in this review were human and animal studies, contain relevant information regarding the neuroprotective action of the drug in PD, applicability of anti-inflammatory drugs, csf analysis, use of in-silico computational method and clinical results and be indexed in the electronic databases MEDLINE/ Pubmed, LILACS, EMBASE, Scopus and Web of Science. Using the PECOS strategy, the descriptors used in the searches were chosen based on the technical-scientific terms MeSH (Medical Subjective Heading) and DeCS (Descriptors in Health Sciences), combined by the Boolean operator “AND” or “OR” (Table 1). MEDLINE/ PubMed research strategy: “Idiopathic Parkinson’s Disease” OR “Lewy Body Parkinson’s Disease” OR “Parkinson’s Disease, Idiopathic” OR “Parkinson Disease, Idiopathic “ OR “Parkinson’s Disease, Lewy Body” OR “Parkinson’s Disease” OR “Idiopathic Parkinson Disease” OR “Lewy Body Parkinson Disease” OR “Primary Parkinsonism” OR “Parkinsonism, Primary” OR “Paralysis Agitans” AND “Neuroinflammation” OR “Inflammations” OR “Innate Inflammatory Response” OR “Inflammatory Response, Innate” OR “Innate Inflammatory Responses” AND “Anti Inflammatory Agents” OR “Agents, Anti-inflammatory” OR “Anti-inflammatories” OR “Anti-inflammatory Agents” OR “Agents, Anti-Inflammatory” OR “Agents, Anti Inflammatory” OR “Anti-Inflammatories” OR “Anti Inflammatories” OR “Anti-inflammatory Agents, Non-Steroidal” OR “NSAIDs” OR “Non-Steroidal Anti-Inflammatory Agents” OR “Non-Steroidal Anti Inflammatory Agents” OR “Nonsteroidal Anti-Inflammatory Agents” OR “Nonsteroidal Anti Inflammatory Agents” OR “Anti Inflammatory Agents, Nonsteroidal” OR “Antiinflammatory Agents, Nonsteroidal” OR “Nonsteroidal Antiinflammatory Agents” OR “Corticosteroids” OR “Corticoids” OR “Inhibitors, Cyclo-Oxygenase” OR “Inhibitors, Cyclo Oxygenase” OR “Inhibitors, Cyclooxygenase” OR “Prostaglandin Synthesis Antagonists” OR “Antagonists, Prostaglandin Synthesis” OR “Inhibitors, Prostaglandin-Endoperoxide Synthase” OR “Inhibitors, Prostaglandin Endoperoxide Synthase” OR “Prostaglandin Endoperoxide Synthase Inhibitors” OR “Prostaglandin Synthase Inhibitors” OR “Cyclo-Oxygenase Inhibitors” OR “Cyclo Oxygenase Inhibitors” OR “Inhibitors, Prostaglandin Synthase” OR “Inhibitors, Cyclooxygenase 2” OR “Cyclooxygenase-2 Inhibitors” OR “Inhibitors, Cyclooxygenase-2” OR “Coxibs” OR “COX-2 Inhibitors” OR “COX 2 Inhibitors” OR “Inhibitors, COX-2” OR “COX2 Inhibitors” OR “Inhibitors, COX2”.
EMBASE research strategy: (‘parkinson disease’/exp/mj OR
‘parkinson disease’/mj OR ‘parkinson`s disease’/mj OR ‘parkinsons
disease’/mj OR ‘paralysis agitans’/mj OR ‘parkinson disease,
symptomatic’/mj) AND (‘anti-inflammatory agent’/exp/mj OR ‘antiinflammatory
agent’/mj OR ‘anti-inflammatory agents’/mj OR ‘antiinflammatory
agents, steroidal’/mj OR ‘anti-inflammatory agents,
topical’/mj OR ‘anti-inflammatory drug’/mj OR ‘anti-inflammatory
agent’/mj OR ‘anti-inflammatory agents’/mj OR ‘anti-inflammatory
agents, steroidal’/mj OR ‘anti-inflammatory agents, topical’/mj OR
‘antiflogistic agent’/mj OR ‘antiinflammation agent’/mj OR ‘anti inflammatory agent’/mj OR ‘anti-inflammatory drug’/mj OR ‘antiinflammatory
steroid’/mj OR ‘anti-inflammatory activity’/exp/mj
OR ‘anti-inflammatory action’/mj OR ‘anti-inflammatory activity’/
mj OR ‘anti-inflammatory effect’/mj OR ‘anti-inflammatory action’/
mj OR ‘anti-inflammatory activity’/mj OR ‘anti-inflammatory
effect’/mj OR ‘antiphlogistic action’/mj OR ‘antiphlogistic activity’/
mj OR ‘antiphlogistic effect’/mj OR ‘nonsteroid anti-inflammatory
agent’/exp/mj OR ‘nsaid’/mj OR ‘anti-inflammatory agents, nonsteroidal’/
mj OR ‘anti-inflammatory agents, non-steroidal’/mj OR
‘anti-inflammatory agent, nonsteroid’/mj OR ‘non steroid antiinflammatory
agent’/mj OR ‘non steroid anti-inflammatory drug’/
mj OR ‘non-steroidal anti-inflammatory agent’/mj OR ‘non-steroidal
anti-inflammatory drug’/mj OR ‘non-steroidal anti-inflammatory
agent’/mj OR ‘non-steroidal anti-inflammatory drug’/mj OR
‘nonsteroid anti-inflammatory agent’/mj OR ‘nonsteroid antiinflammatory
drug’/mj OR ‘nonsteroid antirheumatic agent’/mj
OR ‘nonsteroidal anti-inflammatory drug’/mj OR ‘nonsteroidal
anti-inflammatory drugs’/mj OR ‘nonsteroidal anti-inflammatory
drugs’/mj OR ‘nonsteroidal anti-inflammatory agent’/mj OR
‘nonsteroidal anti-inflammatory drug’/mj OR ‘prostaglandin
synthase inhibitor’/exp/mj OR ‘cyclooxygenase inhibitor’/mj
OR ‘cyclooxygenase inhibitors’/mj OR ‘prostaglandin synthase
inhibitor’/mj OR ‘prostaglandin synthetase inhibitor’/mj OR
‘cyclooxygenase 2 inhibitor’/exp/mj OR ‘cox 2 inhibitor’/mj OR ‘cox
2 specific inhibitor’/mj OR ‘cox 2 specific inhibitors’/mj OR ‘cox-
2 inhibitor’/mj OR ‘cox-2 specific inhibitor’/mj OR ‘cox-2 specific
inhibitors’/mj OR ‘cox2 inhibitor’/mj OR ‘cox2 specific inhibitor’/
mj OR ‘coxib’/mj OR ‘coxibs’/mj OR ‘cyclooxygenase 2 inhibitor’/
mj OR ‘cyclooxygenase 2 inhibitors’/mj) AND (‘modulation’/exp/
mj OR ‘modulation’/mj OR ‘protection’/exp/mj OR ‘protection’/
mj OR ‘protective factors’/mj OR ‘treatment outcome’/exp/mj OR
‘medical futility’/mj OR ‘outcome and process assessment (health
care)’/mj OR ‘outcome and process assessment, health care’/
mj OR ‘outcome management’/mj OR ‘patient outcome’/mj OR
‘therapeutic outcome’/mj OR ‘therapy outcome’/mj OR ‘treatment
outcome’/mj OR ‘disease management’/exp/mj)
LILACS Research Strategy: “Idiopathic Parkinson’s Disease”
OR “Lewy Body Parkinson’s Disease” OR “Parkinson’s Disease,
Idiopathic” OR “Parkinson Disease, Idiopathic “ OR “Parkinson’s
Disease, Lewy Body” OR “Parkinson’s Disease” OR “Idiopathic
Parkinson Disease” OR “Lewy Body Parkinson Disease” OR
“Primary Parkinsonism” OR “Parkinsonism, Primary” OR “Paralysis
Agitans” AND “Neuroinflammation” OR “Inflammations” OR “Innate
Inflammatory Response” OR “Inflammatory Response, Innate” OR
“Innate Inflammatory Responses” AND “Anti Inflammatory Agents”
OR “Agents, Anti-inflammatory” OR “Anti-inflammatories” OR
“Anti-inflammatory Agents” OR “Agents, Anti-Inflammatory” OR
“Agents, Anti Inflammatory” OR “Anti-Inflammatories” OR “Anti
Inflammatories” OR “Anti-inflammatory Agents, Non-Steroidal”
OR “NSAIDs” OR “Non-Steroidal Anti-Inflammatory Agents” OR
“Non-Steroidal Anti Inflammatory Agents” OR “Nonsteroidal
Anti-Inflammatory Agents” OR “Nonsteroidal Anti Inflammatory
Agents” OR “Anti Inflammatory Agents, Nonsteroidal” OR “Antiinflammatory
Agents, Nonsteroidal” OR “Nonsteroidal Antiinflammatory
Agents” OR “Corticosteroids” OR “Corticoids” OR
“Inhibitors, Cyclo-Oxygenase” OR “Inhibitors, Cyclo Oxygenase”
OR “Inhibitors, Cyclooxygenase” OR “Prostaglandin Synthesis
Antagonists” OR “Antagonists, Prostaglandin Synthesis” OR
“Inhibitors, Prostaglandin-Endoperoxide Synthase” OR “Inhibitors,
Prostaglandin Endoperoxide Synthase” OR “Prostaglandin
Endoperoxide Synthase Inhibitors” OR “Prostaglandin Synthase
Inhibitors” OR “Cyclo-Oxygenase Inhibitors” OR “Cyclo Oxygenase
Inhibitors” OR “Inhibitors, Prostaglandin Synthase” OR “Inhibitors,
Cyclooxygenase 2” OR “Cyclooxygenase-2 Inhibitors” OR
“Inhibitors, Cyclooxygenase-2” OR “Coxibs” OR “COX-2 Inhibitors”
OR “COX 2 Inhibitors” OR “Inhibitors, COX-2” OR “COX2 Inhibitors”
OR “Inhibitors, COX2” .
Web of Science Search Strategy
TÓPICO (Parkinson disease*) AND TÓPICO (inflammation*) AND TÓPICO (anti-inflammatory*).
Scopus Search Strategy
(TITLE-ABS-KEY (Parkinson AND disease) AND TITLE-ABSKEY
( inflammation ) AND TITLE ( anti-inflammatory ) ) .
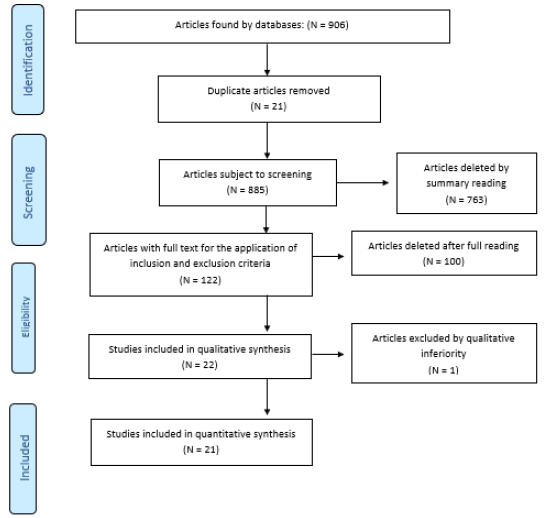
The selection of articles was performed by two researchers
blindly and independently through reading the titles, reading the
abstracts and, finally, full reading of the articles. Any disagreement
in the selection was resolved in consensus meetings. Articles
that fully met the eligibility criteria were included in this study.
The selection process is described in Flowchart 1 adapted from
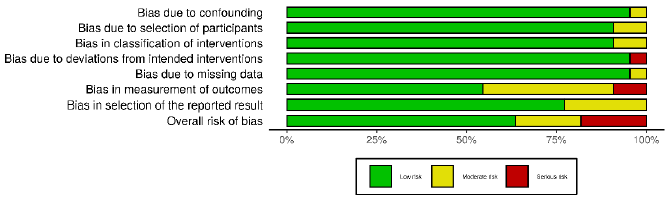
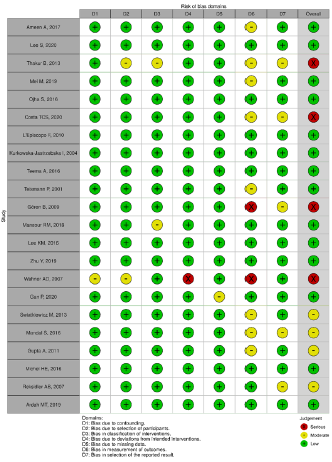
PRISMA (Figure 1). In order to analyze the methodological quality
of the included studies, each article was evaluated by a researcher
based on the items of the ACROBAT-NRSI (A Cochrane Risk of Bias
Assessment Tool for Non-Randomized Studies) [4]. Acrobat-NRSI
scores were used to exclude articles that did not present hardhitting
information to the research, besides serving as a basis
for discussing the methodological quality of the articles and the
possible viruses in the generalization of their results (Figures
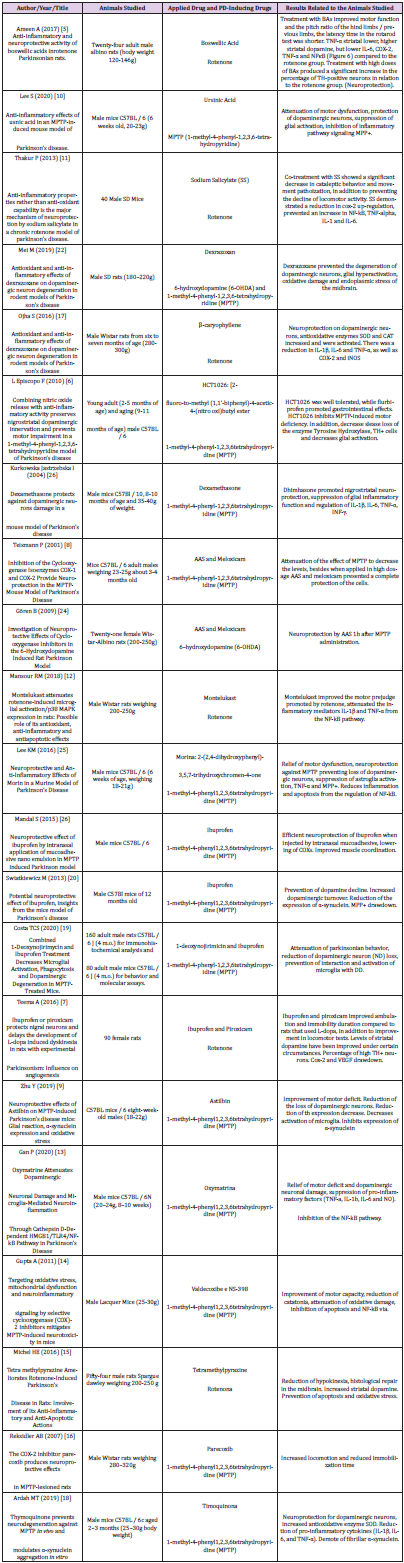
2 & 3). From each article included, data related to the objectives
of this review were extracted, such as author, title, type of study,
population, PD induction drug, drugs used applied, positive results.
These data were computed and compared using the t-Student
test for independent samples, with the purpose of comparing the
percentage s percentages of the and effects on PD between NCAs
and other anti-inflammatory drugs (Table 2).
Findings
Twenty-one articles were analyzed, separated between two groups according to the drug used for pre-clinical study, antiparkinsonian drugs of anti-inflammatory nature and drugs properly anti-inflammatory drugs (IINES and corticosteroids). Improvement in motor function, decreased movement patriotization, increased levels of striatal dopamine, decreased interleukins and blockage of inflammatory pathways, such as those participating in MPP+ and COX-2, as well as increased and/or decreased loss of neurons armed with tyrosine hydroxylase (TH) enzyme, an important marker of neuroprotection, were identified.
Discussion
In view of these findings, this systematic review demonstrated
that there is an effective therapeutic relationship in the use of
anti-inflammatory drugs in PD through findings such as, mainly,
quantitative increase or decrease in the loss of tyrosine hydroxylase
enzyme [5-9]and improvement of motor function or prevention of
motor decline [5,10-16]. However, since these are experimental
studies in animals where clinical failures are commonly recorded
in this methodology, caution should be exercised in the face of
these findings, even if it shows clinical relevance. In addition, the
importance of the therapeutic look is emphasized, especially in
pathophysiological terms elapsed by the articles, observing in
most of them that this disease, which affects the nicrostriatal
region harboring the substantia nigra and quite rich in microglia,
has the cumulative character of alpha synuclein in its altered form,
which leads to the formation of a highly fibrillar aggregate by very
little known pathways, thus, there is the beginning of a cascade
of events that lead to the release of inflammatory toxic factors
and a progressive dopaminergic neurodegeneration [17,18].
It is identified, therefore, that within this pathophysiological
mechanism there is linked an inflammatory response, so there is
a target to be investigated and possibly treated, demonstrating
possible therapeutic purposes against PD.
In parallel, this review was able to investigate some other
parameters found in experimental animal studies. Some motor tests
showed improvement in the face of performance tests, applicability
of previous training or open field observation, in addition, motor
improvement of the forelimbs and later [5], significant decrease
in cataleptic behavior [10], improvement of ambulation and
immobilization time [7]and reduction of hypokinesia [15]. These
results reinforce the hypothesis of a neuroinflammatory cause of
Parkinson’s and once again the application of anti-inflammatory
drugs for a possible therapy. It can be observed that characteristics
that are found in patients such as muscle stiffness, tremor at rest,
bradykinesia and postural instability could be solved or attenuated
by a drug with function, absorption and mechanisms similar to
what were found in this review. Therefore, there is a vast ness of
possibilities for anti-inflammatory pharmacological use, in which, however, there is still a need to weigh the pros and cons, the latter
being something of changeable capacity within the pharmaceutical
industry, in which with investments in research and advanced
technology can be achieved a less deleterious profile to the body,
such as raising blood pressure, interaction with anti-hypertensive
drugs, reduction of renal perfusion and gastrointestinal symptoms
[16].
Within this context, it was also possible to identify an
increase, then neuroprotection from levels of dopamine, TH
enzyme and dopaminergic neurons in some animals. These
results can be explained by the fact that the neuroinflammatory
process, in its characteristic of exponential cascading lesion of
dopaminergic neurons [8,19], was blocked and there was no more
decrease in degenerative character. All this was observed from
immunohistochemical analyses of TH (Tyrosine Hydroxylase)
levels, an enzyme involved in dopamine synthesis through a series
of biochemical reactions that has the amino acid tyrosine as a
precursor and a molecular marker of dopaminergic neurons, along
with dopamine dosage [5-9,18,19]. Thus, it was demonstrated what
can occur in a neural system previously healthy, but with microglia
activated by the pathophysiology of PD, in this case by mimetic
drugs of PD such as rotenone and 1-methyl-4-phenyl-1,2,3,6
tetrahydropyridine (MPTP). Thus, it is envisaged, once again, the
use of these drugs or something more advanced both in patients
already diagnosed and living with the disease chronically, as well as
in patients at the beginning of diagnosis and mild clinical picture,
promoting neuroprotection and, consequently, a greater defense
and increased quality of life.
Some drugs in the studies acted directly on microglia and
other inflammatory foci, some of them are very common, such as
ibuprofen, meloxicam, piroxicam, AAS, Valdecoxib and Parecoxib
(NHEMS, which act by inhibiting COX-2, prostaglandin and
ultimately reducing cytokines), dimethazone (Corticosteroid that
reduces the gene expression of pro-inflammatory cytokines).
All of them obtained good results regarding the lowering of glial
hyperactivation and intracellular inflammatory, in addition to
stimulating the recovery and regeneration phase, avoiding in some
cases the toxicity of MPTP [20], which shows that even having
extensive knowledge and applicability of these drugs, they can still
be key parts for the advancement of neural therapy in PD. Similarly,
oxymatrine, an alkaloid compound found at the root of a Chinese
herb (Sophora flavescent), promoted relief of motor deficits induced
by MPTP and conferred significant neuroprotection, in addition to
inhibiting the activation of microglia and exacerbated release of
pro-inflammatory as cytokines [13]. This shows that within the
vastness of drugs known and disseminated by the pharmaceutical
industry, there are still a gigantic number of other substances that
can be used in the treatment of this disease [20-27].
Conclusion
Our study has concluded that there is a need for investment in quality, more robust, broad-spectrum preclinical studies, with minimal view to achieve the ideal pharmacological therapeutic for this target. Thus, it is necessary more clinic trials to confirm this relationship between an inflammatory profile and use of antiinflammatory drugs which possible therapeutic agents to treatment of PD.
References
- Rocha EM, De Miranda B, Sanders LH (2018) Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiol Dis 109(Pt B): 249-257.
- Pan Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, et al. (2012) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Scientific Reports 2: 898.
- Caffeic T, Phenethyl A, Disease P, Foundation D, October F, et al. (2019) Effects of Caffeic Acid Phenethyl Ester (CAPE) on the turnover of connexins – does it affect alpha-synuclein accumulation in PD models?
- McGuinness LA, Higgins JPT (2020) Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth 12(1): 55-61.
- Ameen AM, Elkazaz AY, Mohammad HMF, Barakat BM (2017) Anti-inflammatory and neuroprotective activity of boswellic acids in rotenone parkinsonian rats. Can J Physiol Pharmacol 95(7): 819-829.
- L Episcopo F, Tirolo C, Caniglia S, Nunzio Testa, Pier A Serra, et al. (2010) Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neuroinflammation 7: 83.
- Teema AM, Zaitone SA, Moustafa YM (2016) Ibuprofen or piroxicam protects nigral neurons and delays the development of l-dopa induced dyskinesia in rats with experimental Parkinsonism: Influence on angiogenesis. Neuropharmacology 107: 432-450.
- Teismann P, Ferger B (2001) Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson's disease. Synapse 39(2): 167-174.
- Ying-Li Zhu, Sun MF, Jia XB, Kun Cheng, Yi Da Xu, et al. (2019) Neuroprotective effects of Astilbin on MPTP-induced Parkinson's disease mice: Glial reaction, α-synuclein expression and oxidative stress. Int Immunopharmacol 66: 19-27.
- Lee S, Lee Y, Ha S, Hae Young Chung, Hangun Kim, et al. (2020) Anti-inflammatory effects of usnic acid in an MPTP-induced mouse model of Parkinson's disease. Brain Research 1730: 146642.
- Thakur P, Nehru B (2013) Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson's disease. Neuroscience 231: 420-431.
- Mansour RM, Ahmed MAE, El-Sahar AE, El Sayed NS (2018) Montelukast attenuates rotenone-induced microglial activation/p38 MAPK expression in rats: Possible role of its antioxidant, anti-inflammatory and antiapoptotic effects. Toxicol Appl Pharmacol 358: 76-85.
- Gan P, Ding L, Hang G, Qiaofang Xia, Zhimei Huang, et al. (2020) Oxymatrine Attenuates Dopaminergic Neuronal Damage and Microglia-Mediated Neuroinflammation Through Cathepsin D-Dependent HMGB1/TLR4/NF-κB Pathway in Parkinson's Disease. Front Pharmacol 11: 776.
- Gupta A, Kumar A, Kulkarni SK (2011) Targeting oxidative stress, mitochondrial dysfunction and neuroinflammatory signaling by selective cyclooxygenase (COX)-2 inhibitors mitigates MPTP-induced neurotoxicity in mice. Prog Neuropsychopharmacol Biol Psychiatry 35(4): 974-981.
- Michel HE, Tadros MG, Esmat A, Khalifa AE, Abdel Tawab AM (2017) Tetramethylpyrazine Ameliorates Rotenone-Induced Parkinson's Disease in Rats: Involvement of Its Anti-Inflammatory and Anti-Apoptotic Actions. Mol Neurobiol 54(7): 4866-4878.
- Reksidler AB, Lima MM, Zanata SM, Machado HB, Da Cunha C, et al. (2007) The COX-2 inhibitor parecoxib produces neuroprotective effects in MPTP-lesioned rats. Eur J Pharmacol 560(2-3): 163-175.
- Ojha S, Javed H, Azimullah S, Haque ME (2016) β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem 418(1-2): 59-70.
- Ardah MT, Merghani MM, Haque ME (2019) Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates α-synuclein aggregation in vitro. Neurochem Int 128: 115-126.
- Costa T, Fernandez Villalba E, Izura V, A M Lucas Ochoa, N J Menezes Filho, et al. (2020) Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J Neuroimmune Pharmacol 16(2): 390-402.
- Swiątkiewicz M, Zaremba M, Joniec I, Członkowski A, Kurkowska Jastrzębska I (2013) Potential neuroprotective effect of ibuprofen, insights from the mice model of Parkinson's disease. Pharmacol Rep 65(5): 1227-1236.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. (2015) Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev 4(1): 1.
- Mei M, Zhou Y, Liu M, Zhao F, Hu G (2019) Antioxidant and anti-inflammatory effects of Dexrazoxane on dopaminergic neuron degeneration in rodent models of Parkinson’s disease. Neuropharmacology 160: 107758.
- Kurkowska Jastrzebska I, Litwin T, Joniec I, Adam Przybyłkowski, Andrzej Członkowski, et al. (2004) Dexamethasone protects against dopaminergic neurons damage in a mouse model of Parkinson's disease. Int Immunopharmacol 4(10-11): 1307-1318.
- Gören B, Mimbay Z, Bilici N, Zarifoğlu M, Oğul E, et al. (2009) Investigation of neuroprotective effects of cyclooxygenase inhibitors in the 6-hydroxydopamine induced rat Parkinson model. Turk Neurosurg 19(3): 230-236.
- Lee KM, Lee Y, Chun HJ, Ah Hyun Kim, Ju Yeon Kim, et al. (2016) Neuroprotective and anti-inflammatory effects of morin in a murine model of Parkinson's disease. J Neurosci Res 94(10): 865-878.
- Mandal S, Das Mandal S, Chuttani K, Krutika K Sawant, Bharat Bhusan Subudhi (2016) Neuroprotective effect of ibuprofen by intranasal application of mucoadhesive nanoemulsion in MPTP induced Parkinson model. Journal of Pharmaceutical Investigation 46(1): 41-53.
- Sarbishegi M, Charkhat Gorgich E A (2019) The Effects of Celecoxib on Rotenone-Induced Rat Model of Parkinson’s Disease: Suppression of Neuroinflammation and Oxidative Stress-Mediated Apoptosis. Gene Cell Tissue 6(2): e92178.

 Review Article
Review Article