Abstract
This study reveals the synthetic method for preparation of the N-arylimidazole derivatives with commercially available aluminium oxy-hydroxide-supported palladium (Pd/AlO (OH)) nanoparticles. The expected products were achieved with high yields in a mixture of H2O/IPA (v/v=1/1) under ultrasonic conditions. It is a province in the literature as the catalyst and application area used. The isopropyl alcohol was used as a support for water for solubility, and reactions were carried out in an environmentally friendly solvent medium. The catalyst purified from the reaction medium was used repeatedly without losing its effectiveness. The method developed for the synthesis of N-arylimidazole derivatives; is an environmentally friendly, economical and highly available method.
Keywords: Pd/AlO(OH) NPs; Heterogenous Catalyst; Coupling; N-Arylimidazole
Introduction
N-arylimidazole derivatives are molecules that have biological activity and have an important place in scientific studies in recent years. The scientists focused on the synthesis of natural and unnatural N-arylimidazole derivatives to examine the intercourse between biological activity and molecular structure [1,2]. Heterocyclic imidazole derivatives such as Liarozole, Alcaftadine and Zolpidem, which are shown in Figure 1, form the active ingredient of many drugs and are known to be vital with their effects. Liarozol has been demonstrated to inhibit cytochrome P450 (CYP) enzymes that perform retinoic metabolism in the body, thereby revealing the body’s retinoic acid synthesis and its therapeutic effect as in synthetic retinoids. Retinoic acids not only support the normal growth of the body, but also play an important role in the differentiation of epithelial cells [3]. Alkaftadine has a therapeutic effect especially on the outermost layer of the eye resulting from an allergic reaction [4]. Zolpidem is used as an active ingredient of drugs used in anxiety and insomnia problems (Scheme 1) [5]. In recent years, the use of heterogeneous catalysts in classical reactions such as hydrogenation [6,7] and coupling [8,9] has become the focus of attention by scientists. Especially the use of single metal containing (monometallic) transition metal nanoparticles as well as two metal containing (bimetallic) nanoparticles as a heterogeneous catalyst creates a more advantageous situation in terms of reaction conditions.
Scheme 1: Active ingredients of some drugs containing imidazole ring some bioactive biphenyl derivatives.
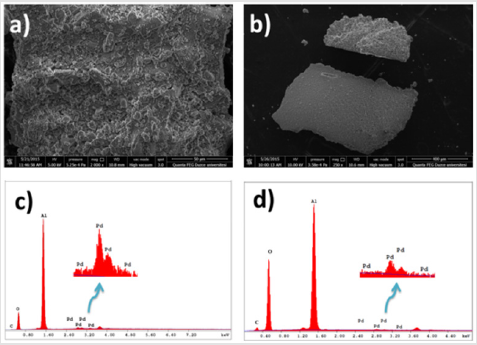
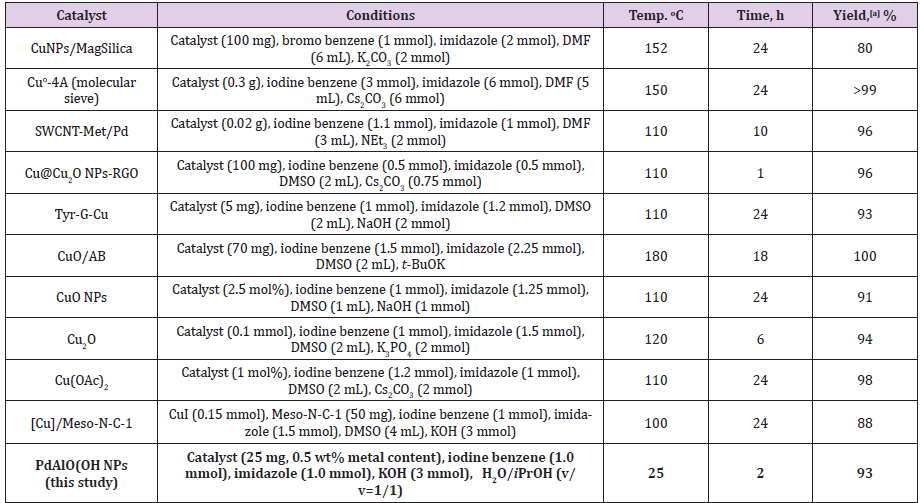
Figure 1: a) SEM image before the reaction;
b) SEM image after five uses;
c) EDX spectrum before the reaction;
d) EDX spectrum after five uses of Pd/AlO(OH) NPs.
There are some disadvantages in the reactions performed with classical methods; high catalyst amounts, low catalyst activity and low reaction efficiency. The more economical catalyst systems are developed with heterogeneous catalysts. Also, heterogeneous catalysts are very easy to obtain, maintain and use. The reuse of the catalysts is an important advantage for heterogeneous catalysts, especially after the reaction, after simple washing and drying with water and some organic solvents, without any significant loss in activity. It is very difficult to remove homogeneous catalysts from the reaction medium and reuse them. Several catalysts such as CuNPs/MagSilica[10] Cuo-4A [11] SWCNT-Met/Pd [12] Cu@Cu2O NPs-RGO [13] Tyr-G-Cu [14]CuO/AB [15]CuO NPs[16]Cu2O[17]Cu(OAc) [2,18]and [Cu]/Meso-N-C-1[19]are utilized for the formation of N-aryimidazol derivatives. On the other hand, due to the high trenchancy of Pd-based catalysts, in the synthesis of N-arylimidazoles has been frequently utilized [20]. In this study, commercially available Pd/AlO(OH) nanoparticles (NPs) were used in the synthesis o [f N-arylimidazoles. Moreover, Pd/AlO(OH) nanoparticles, which maintains its stability in different environmental conditions were used as the catalyst in the hydrogenation of nitro,[6] aldehyde[21] and azido compounds[22] and in the knoevenagle condensation [23] dehalogenation of aryl halides[23] and in the oxidation of benzylic alcohols [24]. Herein, the formation of N-aryl imidazoles was carried out using ultrasonic conditions and PdAlO(OH) NPs, which is highly stable and reusable in eco-friendly solvent mixture such as H2O / IPA.
Experimental
Materials
hydroxide) and solvents were provided from Sigma-Aldrich and used without further purification.
Characterization Methods
1H/13C NMR spectra were recorded on a Jeol ECS 400 MHz spectrometer.All reagents (imidazoles, aryl halides, catalyst, potassium
General Procedure for the Synthesis of N-Arylimidazoles
1.0 mmol of imidazole compounds and 3.0 mmol of potassium hydroxide with 4 ml of water-isopropyl mixture (v / v = 1/1) in the reaction vessel were mixed with the magnetic stirring for 15 minutes. Then, 25 mg of PdAlO (OH) NPs and 1.0 mmol of aryl halides were added to the mixture, and the reaction was continued under ultrasonic conditions for 2 hours. After completion of the reaction, the catalyst was removed by applying simple centrifugation and washing with ethanol (3x15 ml). The filtrate was taken to dryness in the evaporator and N-aryl imidazole derivatives were purified by silica gel column chromatography with ethyl acetate / hexane mixture (v/v=1/5).
Results and Discussion
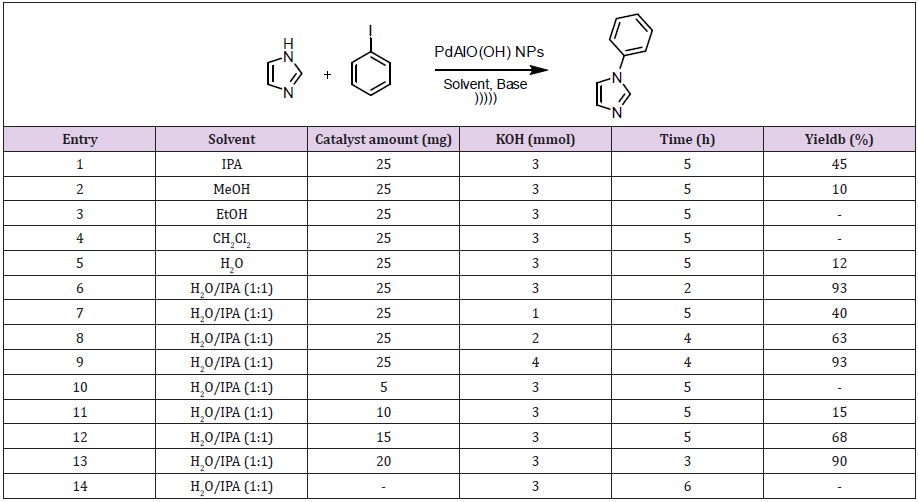
The Scanning Electron Microscopy (SEM) and x-ray diffraction (XRD) (Figure S1) analysis of the catalyst was taken before the synthesis of N-aryl imidazole derivatives and after the same reaction was repeated five times. As seen in irregularly spreading SEM images, the catalyst displays the nanocrystalline Boehmite structure, a hydrated form of alumina (AlOOH). When the surface area of a nanocatalyst is sufficiently enlarged, information about the surface of the catalyst can be obtained (Figure 1). After the Pd/ AlO (OH) nanoparticles are reused five times as shown in Figure 1b, agglomerates occur on the catalyst surface. The elemental composition of the catalyst consists of carbon, aluminum and palladium, as seen in Figures 1c & 1d. It is likely that carbon is contaminated by air. In the literature, XRD analysis of Pd/AlO(OH) nanoparticles clearly reveals the surface-centered cubic crystal structure of the catalyst [25]. The catalytic activity of Moreover, Transmission Electron Microscopic Analysis (TEM) reveals that the average size of Pd/AlO(OH) NPs is about 3 nm [26] the Pd/ AlO(OH) NPs was studied for the synthesis of N-phenyl imidazole in the presence of KOH as base source under ultrasonic conditions (Table 1). Firstly, different solvents such as isopropyl alcohol (IPA), methanol (MeOH), ethanol (EtOH), dichloromethane (CH2Cl2) and water (H2O) were tested.
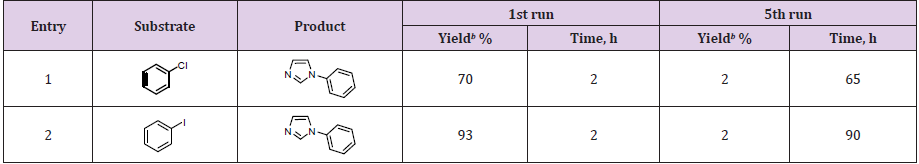
The best results were obtained by the using of water/isopropyl as solvent mixture. The compatibility of water with the substrate and product was also noteworthy. Addition of 3.0 mmol of KOH with 15 mg of Pd/AlO(OH) NPs as catalyst in the presence of H2O/ IPA mixture showed a significant increase in the yield (Table 1, entry 12). Eventually, 25 mg of catalyst and 3.0 mmol of KOH were used with 4.0 ml of water/isopropyl alcohol (v/v=1/1) to obtain N-phenyl imidazole (Table 1, entry 6). However, there was no N-phenyl imidazole formation in the absence of catalyst and under ultrasonic conditions (Table 1, entry 14). Table 2 showed that the commercially available Pd/AlO(OH) catalyst was tested in the synthesis of N-aryl imidazole derivatives. In this reaction, aryl halides and imidazole derivatives were preferred as the starting material. N-phenyl imidazole (2) and 1-(4-nitrophenyl)- 1H-imidazole (6) compounds were obtained using different aryl halides. In this study, the effects of the groups –Cl, -Br and –I were compared as the leaving group. As expected, iodine benzene (4) reacted with higher yields to obtain related products (Table 2, entries 1-6).
1-(4-Bromophenyl)-4-nitro-1H-imidazole (10) was obtained in low yields. Because, the nucleophilic feature is weakened due to the -NO2 group, which is the electron attracting group in the imidazole structure used as the starting material (Table 2, entry 7). The presence of the -NO2 group in the imidazole structure and the presence of the -MeO group in the aryl halide structure caused the formation of 1-(4-Methoxyphenyl)-4-nitro-1H-imidazole (12) in lower yields (Table 2, entry 8). In the formation of 1- (4-Methoxyphenyl)-2-methyl-4-nitro-1H-imidazole (13), in addition to the aforementioned electronic effects, steric effects also manifested and the reaction efficiency decreased even more (Table 2, entry 9). The electronic effects come to the fore in the formation of 4-ethoxycarbonyl-1- (2-nitrophenyl) imidazole (15), 4-ethoxycarbonyl-1-(4-methylphenyl) imidazole (17) and 4-ethoxycarbonyl-1-(2-chlorophenyl) imidazole (19) compounds. As expected, aryl halides containing nitro groups turn into products with higher yields (Table 2, entries 10-12).The amount of palladium passed to the reaction medium after 5 repeated reactions in the water-isopropyl solvent mixture under ultrasonic conditions was calculated with ICP-MS and is 1%. This result is a negligible result, suggesting that the method is an environmentally friendly method (Table 3).
The literature information related to the synthesis of N-phenyl imidazole is given in Table 4. The use of different types of heterogeneous catalysts in N-arylation reactions appears to have some advantages: high yield synthesis of products, recovery of catalysts and repeated use. However, it is an important problem that the reaction times are long and the reactions are carried out at high temperatures. Table 4 shows that copper-containing heterogeneous catalysts are used in N-arylation reactions. However, since the catalysts containing copper are unstable, the catalyst structure deteriorates under atmospheric conditions and the transition of the metal to the reaction medium is an environmental threat. Therefore, the repeated use of copper-containing catalysts is very weak. However, the catalyst we use in this study is stable under different atmospheric conditions and has a feature that can be used many times by being easily removed from the reaction medium. Of course, in the literature, heterogeneous catalysts containing palladium are also used in N-arylation reactions. However, as seen in Table 1, long reaction times and high temperatures are a major disadvantage in terms of time and energy economy.
Table 4: Comparison of designed catalytic system with recent published works about the synthesis of N-phenylimidazole.
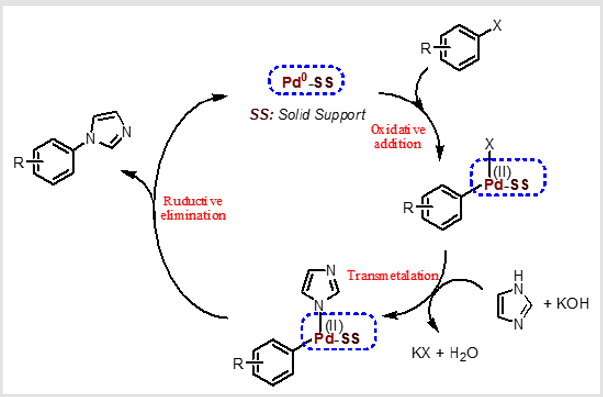
Also, the use of toxic and carcinogenic solvents such as DMF and DMSO is a negative side of N-arylation reactions in the literature. However, as in different catalytic applications, the main purpose in catalytic applications of catalysts is to develop a more advantageous method. The main purpose of our study is to give the literature an economical method for the synthesis of N-aryl imidazoles in an environmentally friendly solvent, accompanied by a heterogeneous catalyst that is commercially available, recyclable and reused. The scheme of the coupling reactions of aryl halides and imidazole derivatives occurring on the catalyst surface is given in Scheme 2. In the proposed mechanism; After AlO(OH) supported Pd (0) was oxidized to Pd (II) with oxidative addition step, N-arylimidazole products were obtained with the formation of steps such as transmetalation and reductive elimination In the transmetalation phase, KOH, a strong base, accelerates the separation of the hydrogen atom from the imidazole compound. In this way, the nucleophilic power of the imidazole molecule increases and the coupling reaction with aryl halides is accelerated over the catalyst surface. Finally, Pd (II) is reduced to Pd (0) again and the reaction continues under catalytic conditions [27].
Conclusion
In summary, a new method has been developed for the synthesis of N-aryl imidazole derivatives with high yields and short periods using Pd/AlO(OH) NPs in ultrasonic conditions. The use of the relevant catalyst in coupling reactions is a first in the literature. The use of a second solvent, such as isopropyl alcohol, which will support the solubility of imidazole derivatives and aryl halides, as the starting materials, increases the solubility and makes the reaction easily. In addition, after the reaction, the catalyst was gained and used again and again. The rate of metal that has passed into the solvent after their reuse was determined with ICP-MS. The method we developed is an alternative for environmentally friendly, economical and docking reactions.
Acknowledgment
This research was supported by Duzce University Research Fund (grant no. 2019.26.04.979).
Competing-Interest
The author(s) declare no competing interests.
References
- MA Bonin, D Giguere, R Roy(2007) Tetrahedron 63: 4912-4917.
- SN Mokale, D Lokwani, DB Shinde, Bioorg (2012) Synthesis, Biological Activity and Docking Study of imidazol-5-one as Novel Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. Med Chem 20: 3119-3127.
- HM Bryson, AJ Wagstaff (1996) Drugs & Aging 9: 478-484.
- R Namdar, C Valdez (1998) Alcaftadine: A Topical Antihistamine for Use in Allergic Conjunctivitis Drugs of Today 47: 883-890.
- TS Harrison, CM Keating (2005) Current awareness in human psychopharmacology. CNS Drugs 19(6): 65-89.
- H Goksu (2015) Recyclable aluminium oxy-hydroxide supported Pd nanoparticles for selective hydrogenation of nitro compounds via sodium borohydride hydrolysis. New J Chem 39: 8498-8504.
- MB Gawande, H Guo, AK Rathi, PS Branco, Y Chen (2013) First application of core-shell Ag@Ni magnetic nanocatalyst for transfer hydrogenation reactions of aromatic nitro and carbonyl compounds. RSC Adv 3(4): 1050-1054.
- A Fodor, Z Hell, L Pirault Roy (2014)Efficient C–C cross-coupling reactions by (isatin)-Schiff base functionalized magnetic nanoparticle-supported Cu(II) acetate as a magnetically recoverable catalyst Appl. Catal A 484: 39-50.
- R Ghorbani Vaghei, S Hemmati, H Veisi (2014) Pd immobilized on amidoxime-functionalized Mesoporous SBA-15: A novel and highly active heterogeneous catalyst for Suzuki–Miyaura coupling reactions. J Mol Catal A 393: 240-247.
- F Nador, MA Volpe, F Alonso, G Radivoy(2014) Tetrahedron 70: 6082-6087.
- J Németh, N Debreczeni, I Gresits, M Bálint, Z. Hell (2015) Catal Lett145: 1113-111.
- H Veisi, N Morakabati (2015) Palladium nanoparticles supported on modified single-walled carbon nanotubes: a heterogeneous and reusable catalyst in the Ullmann-type N-arylation of imidazoles and indolesNew J Chem 39(4): 2901-2907.
- SK Movahed, M Dabiri, A Bazgir(2014) Realization of Tunable Localized Surface Plasmon Resonance of Cu@Cu2O Core–Shell Nanoparticles by the Pulse Laser Deposition MethodAppl Catal A 481: 79-88.
- Q Huang, L Zhou, X Jiang, Y Zhou, H Fan (2014) Synthesis of Copper Graphene Materials Functionalized by Amino Acids and Their Catalytic Applications. ACS Appl Mater Interfaces 6(16): 13502-13509.
- AY Kim, HJ Lee, JC Park, H Kang, H Yang (2009)Highly Efficient and Reusable Copper-Catalyzed N-Arylation of Nitrogen-Containing Heterocycles with Aryl Halides. Molecules14(12): 5169-5178.
- L Rout, S Jammi, T Punniyamurthy(2007) Novel CuO Nanoparticle Catalyzed C−N Cross Coupling of Amines with IodobenzeneOrg Lett9(17): 3397-3399.
- Y Wang, J Luo, Z Liu(2013) Synthesis, characterization and cytotoxicity of Pt(II), Pd(II), Cu(II) and Zn(II) complexes with 4’‐substituted terpyridine. Appl Organomet Chem 27(3): 601-605.
- ZL Xu, HX Li, ZG Ren, WY Du, WC Xu (2011) Cu(OAc)2H2O-catalyzed N-arylation of nitrogen-containing heterocycles. Tetrahedron 67(29): 5282-5288.
- P Zhang, J Yuan, H Li, X Liu, X Xu (2013) RSC Adv 3: 1890-1895.
- S Priyarega, DS Raja, SG Babu, R Karvembu, T Hashimoto (2012) Polyhedron 34: 143-148.
- H Goksu, Y Yıldız, B Çelik, M Yazıcı, B Kilbas (2016) Eco-friendly hydrogenation of aromatic aldehyde compounds by tandem dehydrogenation of dimethylamine-borane in the presence of a reduced graphene oxide furnished platinum nanocatalyst. Catal Sci Technol 6(7): 2318-2324.
- BY Kara, B Kilbas, H Goksu(2016) Palladium Nanoparticles Entrapped in Aluminum Hydroxide: Dual Catalyst for Alkene Hydrogenation and Aerobic Alcohol OxidationNew J Chem 40: 9550-9555.
- H Göksu, E Gültekin(2017) Chem Select 2: 458-463.
- H Goksu, F Sen (2020) Sci Rep 10: 5731-5737.
- H Hou, Y Zhu, Q Hu(2013) Synthesis of Novel Pd/γ-AlOOH Composites and Its Electrooxidation toward Methanol in Alkaline Solution. Mater Trans 54: 1686-1690.
- MS Kwon, N Kim, CM Park, JS Lee, KY Kang (2005) Heterogeneous Copper Catalyst for the Cycloaddition of Azides and Alkynes without Additives under Ambient Conditions. Org Lett 7: 1077-1079.
- J Xia, Y Fu, G He, X Sun, X Wang (2017) Pd-doped Ni nanoparticle-modified N-doped carbon nanocatalyst with high Pd atom utilization for the transfer hydrogenation of nitroarenes. Appl Catal B 200: 39-46.

 Research Article
Research Article