Abstract
Increasing evidence proposes that inflammation play a vital role in the pathogenesis of osteoarthritis (OA).The nucleotide-binding oligomerization domain (NOD)-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome has been found to be involved in OA pathogenesis by activation of Toll-like receptors (TLR) and NF-κB signal pathway, thus enhancing the production of proinflammatory cytokines and subsequently resulting in cartilage degeneration and synovial inflammation. Here, we provide an overview of the molecular mechanism and regulation of the NLRP3 inflammasome and its role in the pathogenesis of OA. Finally, we contemplate the potential of therapeutic targeting to the NLRP3 inflammasome and its associated signaling pathways as a promising way for OA treatment.
Abbreviations: OA: Osteoarthritis; NLRP: Nucleotide-Binding oligomerization domain (NOD)-like receptor family, Pyrin Domain-Containing 3; DAMPs: Danger-Associated Molecular Patterns ;PAMPs: Pathogen-Associated Molecular Patterns; ASC: Apoptosis- Associated Speck-like Protein a Caspase Recruitment Domain; PRRs: Pattern Recognition Receptors; DMM: Destabilization of the Medial Meniscus; Nrf2: Nuclear Factor E2- Related factor 2; TLRs: Toll like Receptors
Introduction
Osteoarthritis (OA), the most common form of joint disease among the elderly, is characterized by irreversible articular cartilage destruction and secondary synovial inflammation. It was estimated that the prevalence of OA among adults in the United States is about 12%, and this number is expected to increase due to an ageing population [1]. Inflammasome could enhance inflammatory responses by improving the level of key cytokines significantly in an inflammation process. The NLRP3 inflammasome acts as a pivotal player in the pathogenesis of several arthritic disorders such as rheumatoid arthritis (RA), gouty arthritis, and OA. Many researchers have observed that the activated NLRP3 inflammasome can induce the production and release of interleukin-1β (IL-1β), IL-18, and TNF-α those pro-inflammatory cytokines driven cartilage degeneration via NF-κB signal pathway [2] In this review, we aim to summarize the activation and regulation of NLRP3 inflammasome, and discuss its role in the progression of OA.
NLRP3 Inflammasome
The NLRP3 inflammasome belongs to a subfamily of the NLR family, which is a multiprotein complexes comprised of NLRP3, apoptosis-associated speck-like protein a caspase recruitment domain (CARD) (ASC), and procaspase-1 [3]. NLRP3 as a receptor protein in the complexes can be triggered by danger-associated molecular patterns (DAMPs) and/or pathogen-associated molecular patterns (PAMPs) which are recognized by pattern recognition receptors (PRRs) [4,5]. Two steps for NLRP3 activation are commonly recognized. The priming step (first step) through the nuclear factor‐κB (NF-κB) pathway, which is induced by inflammatory stimuli (such as TLR ligands or endogenous cytokines) resulting in the expression of NLRP3 and pro-IL-1β. The activating step (second step) initiates NLRP3 oligomerization, thus leading the assembly of NLRP3, ASC and pro-caspase-1 to form the inflammatory, and ultimately promote the maturation and secretion of IL-1β and IL-18 [6,7].
NLRP3 Inflammasome-Mediated Inflammatory Pathways in OA
Accumulating evidence highlights a key role for the NLRP3 inflammasome in the pathomechanism of OA. Studies using mouse surgical destabilization of the medial meniscus (DMM) model of OA demonstrated the enhanced expression of NLRP3 and procaspase- 1, along with elevated proinflammatory cytokines such as IL-1β and TNF-α released by chondrocytes and macrophages in mice arthrodial cartilage, compared to no surgery and sham controls [8]. In other studies, both in vitro and in vivo data demonstrated that the activation of NLRP3 inflammasome was dependent on ROS-mediated the nuclear factor E2-related factor 2 (Nrf2)/ heme oxygenase 1(HO-1) signaling. This indicates that ROS is a driver of OA pathology via activating NLRP3, which is involved in OA cartilage degradation by producing IL-1β and IL-18 [9].
NLRP3-Based Inhibitors for OA Treatment
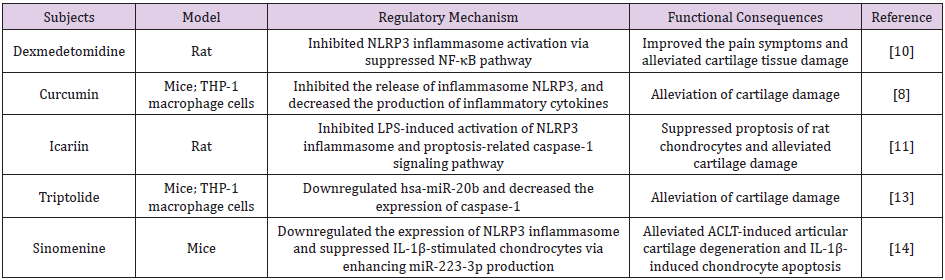
Given the primary role of NLRP3 inflammasome activation in OA progression, targeting NLRP3 or its regulators may be a promising option for new drug development. Then, pre-clinical studies investigating application of various NLRP3 inhibitors for treating OA are summarized and critically analyzed (Table 1). For example, dexmedetomidine, a highly selective α2 receptor agonist, suppressed NLRP3 inflammasome activation significantly in OA rat model induced by papain through inhibiting NF-κB pathway [10]. Icariin is extracted from Epimedium, which could alleviate pyroptosis and subsequently protect against cartilage tissue damage via NLRP3-caspase-1 signaling pathway [11]. Similarly, curcumin has shown protective potential on OA by inhibiting NALP3/caspase-1 activation and decreasing pro- and mature IL-1β generation [8].
Table 1: NLRP3 participated in the regulation of OA in different models.
Note: LPS: lipopolysaccharide; ACLT: Anterior Cruciate Ligament Transection.
Micro RNA-Based Therapy: Micro RNA (miRNA) is a class of short, non-protein coding RNA molecules, which can form ribonucleoprotein complexes and eventually induce degradation of the specific mRNA. Numerous studies indicated that upregulation of miRNAs suppressed NLRP3 inflammasome activation at the level of transcription or translation [12]. Therefore, targeting miRNA may provide a new therapeutic avenue for OA treatment. Triptolide, as the main biologically active component of Tripterygium wilfordii, could downregulate hsa-miR-20b and downstream pathway by decreasing the expression of NLRP3 inflammasome [13]. Sinomenine, another miRNA-based inhibitor, similarly downregulated the expression of NLRP3 inflammasome in OA mice and suppressed IL-1β-stimulated chondrocytes via enhancing miR- 223-3p production [14].
ROS-based Therapy: A series of recent studies shown that ROS might be correlated with NLRP3 inflammasome activation. These studies used various antioxidants for treating NLRP3 inflammasome-associated diseases such as cardiovascular and brain diseases via scavenging excessive ROS and subsequently suppressing NLRP3 inflammasome activation [15]. However, few studies explore the relationship between antioxidants, NLRP3, and OA treatment. In our previous study [16], we have found catalase, a kind of antioxidant, could enhance cell viability and counteract TNF-α induced apoptosis. In addition, study to further explore the relationship between CAT and NLRP3 is ongoing.
Conclusion
Being an innate immune cell sensor, the NLRP3 inflammasome plays a primary role in OA development. Some studies have explored the therapeutics that target the NLRP3 and its regulators. Nevertheless, additional research is necessary to fully elucidate mechanisms behind the inflammasome, which may result in better treatments.
Acknowledgement
This work was financially supported by Guangzhou 121 Echeloned Promoting Project for Talented Scientists [SRF (2009) 149].
References
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, et al. (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis & Rheumatism 58(1): 26-35.
- Chen L, Lan Z (2017) Polydatin attenuates potassium ozonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food & function 8(5): 1785-1792.
- Von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE, et al. (2013) Recognition of bacteria by inflammasomes. Annual review of immunology 31: 73-106.
- Marina-García N, Franchi L, Kim YG, Miller D, McDonald C, et al. (2008) Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. The Journal of Immunology 180(6): 4050-4057.
- Takakubo Y, Barreto G, Konttinen YT, Oki H, Takagi M, et al. (2014) Role of innate immune sensors, TLRs, and NALP3 in rheumatoid arthritis and osteoarthritis. Journal of long-term effects of medical implants 24(4): 243-251.
- Liu Q, Zhang D, Hu D, Zhou X, Zhou Y, et al. (2018) The role of mitochondria in NLRP3 inflammasome activation. Molecular immunology 103: 115-124.
- Meng QQ, Feng ZC, Zhang XL, Hu LQ, Wang M, et al. (2019) PPAR-γ Activation Exerts an Anti-inflammatory Effect by Suppressing the NLRP3 Inflammasome in Spinal Cord-Derived Neurons. Mediators of inflammation pp. 6386729.
- Sun Y, Liu W, Zhang H, Li H, Liu J, et al. (2017) Curcumin prevents osteoarthritis by inhibiting the activation of inflammasome NLRP3. Journal of Interferon & Cytokine Research 37(10): 449-455.
- Chen Z, Zhong H, Wei J, Lin S, Zong Z, et al. (2019) Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Research & Therapy 21(1): 1-13.
- Cheng F, Yan FF, Liu YP, Cong Y, Sun KF, et al. (2019) Dexmedetomidine inhibits the NF-κB pathway and NLRP3 inflammasome to attenuate papain-induced osteoarthritis in rats. Pharmaceutical biology 57(1): 649-659.
- Zu Y, Mu Y, Li Q, Zhang ST, Yan HJ, et al. (2019) Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated proptosis. Journal of orthopedic surgery and research 14(1): 307.
- Yang Z, Zhong L, Xian R, Yuan B (2015) MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Molecular immunology 65(2): 267-276.
- Qian K, Zhang L, Shi K (2019) Triptolide prevents osteoarthritis via inhibiting hsa-miR-20b. Inflammopharmacology 27(1): 109-119.
- Dong HC, Li PN, Chen CJ, Xu X, Zhang H, et al. (2019) Sinomenine attenuates cartilage degeneration by regulating miR-223-3p/NLRP3 inflammasome signaling. Inflammation 42(4): 1265-1275.
- Zeng J, Chen Y, Ding R, Feng L, Fu Z, et al. (2017) Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS-and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. Journal of neuroinflammation 14(1): 119.
- Li S, Yang X, Feng Z, Wang P, Zhu W, et al. (2018) Catalase enhances viability of human chondrocytes in culture by reducing reactive oxygen species and counteracting tumor necrosis factor-α-induced apoptosis. Cellular Physiology and Biochemistry 49(6): 2427-2442.

 Mini Review
Mini Review