Research Article
Regulation of Murine Myometrial Contraction by
Ginger Extract Via Activation of Voltage Dependent
Ca2+ Channels
Seung Hwa Hong1, Kyu-Sang Kyeong2, Il Woon Ji1, Eun-Hwan Jeong1, Hak Soon Kim1, Bang Yeon Hwang3, Chul Lee3, Seung Myeung Son4, Young Chul Kim5*, Ra Young You5, Sang Jin Lee5, Kyuen Na6,
Soyoung Ahn6, Ji Young Park7, Chan Hyung Kim8, Hun Sik Kim8, Woong Choi8, Yeon Jin Park9 and
Wen-Xie Xu10
Author Affiliations
1Department of Obstetrics and Gynecology, College of Medicine, Chungbuk National University(CBNU), Chungdae-ro 1,
Seowon-gu, Cheongju, Chungbuk, 28644, Korea
2Department of Obstetrics and Gynecology, Hallym Univ, Dongtam, Sared Heart Hospital, Heaseong, Korea
3Department of Pharmacy, CBNU, Cheongju, Chungbuk, 28644, Korea
4Department of Pathology, CBNU, Cheongju, Chungbuk, 28644, Korea
5Department. of Physiology, College of Medicine, CBNU, Cheongju, Chungbuk, 28644, Korea
6A Medical student, College of Medicine, CBNU, Cheongju, Chungbuk, 28644, Korea
7Deptartment of Psychiatry, CBNU, Cheongju, Chungbuk, 28644, Korea
8Dept. of Pharmacology, College of Medicine, CBNU, Cheongju, Chungbuk, 28644, Korea
9Department of Obstetrics and Gynecology, Chengju ST. Mari’s Hospital, Cheonwon-Gu, Jusung-Ro, Cheongju, Chungbuk, 173-19, Korea
10Department of Physiology, College of Medcine, Shanghai Jiaotong University, 800 Dongchun Rd. Shanghai, 200240, P.R. China
Received: August 09, 2019 | Published: August 21, 2019
Corresponding author: Woong Choi, Department of Pharmacology, Chungbuk National University, College of Medicine,
Chungdae-ro 1, Seowon-Gu, Cheongju 28644, Korea
Corresponding author: Woong Choi, Department of Pharmacology, Chungbuk National University, College of Medicine,
Chungdae-ro 1, Seowon-Gu, Cheongju 28644, Korea
DOI: 10.26717/BJSTR.2019.20.003499
Voltage-dependent L-type Ca2+ channel (VDCCL) and T-type Ca2+ channel (VDCCT)
in murine myometrium was identified in murine myometrium. Its regulatory functions
were characterized by using extracts of ginger. Methanol extract of ginger was used
to obtain dichloromethane fraction (Gin C). Spontaneous uterine contractions were
enhanced by BayK 8644, a VDCCL activator. However, such effects were inhibited by
nifedipine (a VDCCL blocker) and mibefradil (a VDCCT blocker). Mibefradil also inhibited
oxytocin (OXT), prostaglandins F2α (PGF2α), and prostaglandins E2 (PGE2)-induced
contractions. However, application of BayK 8644 in the presence of mibefradil recovered
those contractions in a nifedipine-sensitive manner. These results suggest that both
VDCCL and VDCCT are important in the regulation of murine myometrial contractions.
Gin C (200 mg/mL) completely inhibited spontaneous contractions of murine uterus
reversibly. The inhibition by Gin C on spontaneous contractions independent of L-NAME,
K+ channel blockers, and nerve blockers. High K+ (50 mM)-induced contraction in the
presence and absence of cyclopizonic acid (CPA) was also completely inhibited by Gin
C, respectively. In addition, Gin C inhibited oxytocin (OXT; 10 nM)-induced contraction
independent of L-NAME and blockers of protein kinases. Prostaglandin F2α (PGF2α)- and
acetylcholine (ACh) produced contractions were also inhibited by Gin C. These results
raise the possibility that Ginger extracts C inhibits spontaneous, high K+-, OXT-, PGF2α-
and ACh-induced contractions by inhibition of VDCCL in mouse uterine longitudinal
smooth muscle.
Keywords: Myometrium; VDCCL; VDCCT;
Oxytocin; Ginger
Ginger (Zingiber officinae Roscoe) is a flowering plant in family
Zingiberaceae whose roots is widely used as an ingredient in both
cooking and East Asian traditional medicine. Ginger is traditionally
used to treat fever, nausea, vomiting, and uterine disorders. Ginger
has also been used to treat paralytic ileus in Japan [1-4]. It has been reported that ginger can regulate contractility of uterine,
gastrointestinal (GI) tract, and airway smooth muscle. Rat uterine
muscle tone and spontaneous contractions can also be enhanced
by ginger extract [5]. Such enhancing effect on the contraction is
produced by activation of voltage-dependent L-type Ca2+ channels
(VDCCL) and release of Ca2+ from sarcoplasmic reticulum [5].
Other reports have suggested similar enhancing effects of ginger
extract on rabbit uterine smooth muscle tone and its spontaneous
contractions [6]. In general, VDCCL is essential for the regulation
of smooth muscle contractility in many species [7-10]. Nifedipine,
a VDCCL blocker, can also block oxytocin (OXT)-induced phasic
contraction in murine myometrium (unpublished data). Regulatory
effects of ginger on contractility of uterine, gastrointestinal (GI)
tract, and airway smooth muscle have been reported [5-6,11].
Ginger also can improve gastric emptying and motility,
intestinal contractility, and irritable bowel syndrome (IBS) in the
GI tract [12-13]. In addition, ginger extract and its components can
also inhibit motility of GI including the lower esophageal sphincter
(LES) [14-15]. Other studies have suggested that ginger extract has
regulatory effects on airway and vascular smooth muscle [16-17].
However, the mechanism of action involved in the effect of ginger
extract on these organs remains unclear. In this study, we found that
ginger extract could inhibit uterine smooth muscle contractility by
blocking VDCCs. Since ginger is safe for humans, ginger might have
potential to be developed as a tocolytic agent to relieve excessive
uterine contractions.
Tissue Preparation for Isometric Contraction
All experiments were performed in accordance with the
guidelines for animal care and use provided by Chungbuk
National University (CBNUA-383-11-01; CBNUA-597-13-02;
CBNUA-719-14-01; CBNUA-863-15-01; CBNUA-988-16-01;
CBNUA-1125-17-02; CBNUA-1162-18-02). All animal experiments
were conducted in accordance with the National Institutes of Health
(USA) Guidelines for the Care and Use of Laboratory Animals (the
Guide for the Care and Use of Laboratory Animals (8th edition,
National Academies Press)) and were approved by the Chungbuk
National University Medical School Research Institutional Animal
Care and Use Committee (Korea). Female non-pregnant mouse
was used in this whole study. Institute of Cancer Research (ICR)
mice (age, 10–12 weeks) were anaesthetized with fluoromethyl
2,2,2-trifluoro-1(trifluoromethyl) ethyl ether (Sevoflurane;
Maruishi Pharma., Osaka, Japan) and/or chloroform and killed by
cervical dislocation. Their uteri were cut open from the neck to the
end of uterine horns, rinsed in Krebs-Ringer bicarbonate (KRB)
solution, and pinned on a Sylgard plate to maintain their original
shape. Connective tissues were removed, and these uteri were cut.
The endometrium was separated from other muscle layers in
KRB solution. Longitudinal muscle strips (1 × 5 mm) were mounted
onto vertical chambers (25 and 75 mL) in an isometric contractile
measurement system with one end of the tissue tied tightly to
a fixed holder while the other end of the tissue was linked to a
force transducer (Harvard Instruments, Holliston, MA, USA) by a
hook. The force transducer was connected to a Power Lab-Data
Acquisition System and a personal computer running Charter v5.5
software (ADinstruments, Boulder, CO, USA) to measure isometric
contractions. Each strip was stretched passively to resting tension
for 1-2 hours after equilibration for 1.5 hours. Contractile responses
of the strip to high K+ (50 mM) were repeated twice.
Ginger Extraction
Dried ginger powder was purchased from a local company
and 300 g was extracted twice with methanol for 2 L 24 hours at
room temperature. Four liters of this methanol extract was dried
in a rotary evaporator to yield 21 g of precipitate. The precipitate
was resuspended in 500 ml of water and mixed with 500 ml of
dichloromethane. The mixture was set at room temperature until
water and dichloromethane phases separated clearly. Water and
dichloromethane fractions were then collected and freeze-dried.
Yields of water and dichlromethane fractions were 9.4 g and
11.4 g, respectively. We already got patent in Korea (Patent No:
1018089440000; Title: Composition for preventing and treating
dysmenorrhea and premature labor comprising non-polar solvent
subfraction from Zingiber officinale extract; Web site: http://
engportal.kipris.or.kr/engportal/search/total_search.do)
Solution and Drugs
KRB solution (CO2/bicarbonate-buffered Tyrode) contained (in
mM) the following: 122 mM NaCl, 4.7 mM KCl, 1 mM MgCl2, 2 mM
CaCl2, 15 mM NaHCO3, 0.93 mM KH2PO4, and 11 mM glucose (pH
7.3-7.4, bubbled with 5 % CO2/95 % O2). Equimolar concentration
of Na+ was replaced with K+ to make high K+ (50 mM) solution. The
external solution was changed with solution that had been bubbled
with 5% CO2/95% O2, 36 °C) in a water bath before application.
Various blockers were applied for 12-15 minutes before
application of Gin C. Then a K+ channel blocker cocktail (KBC)
was applied before application of stimulators to block each K+
channel’s responses. KBC contained 4-aminopyridine (4-AP, 2 mM),
tetraethylammonium (TEA, 5 mM), apamin (APA, 300 nM), and
glibenclamide (Glib, 20 μM). To rule out nerve mediated response, a
nerve blocker cocktail (NBC) was used. NBC contained tetrodotoxin
(TTX, 0.4 μM), guanethidine (1 μM), and atropine (ATR, 1 μM) [7-9].
All drugs used in this study were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Statistics
Data are expressed as means ± standard errors of the mean
(SEM). Statistical significance was measured using analysis of
variance (ANOVA) and student’s t-test. Any p-value less than 0.05
was regarded as statistically significant.
StatIsometric Contraction of Mouse Uterine Longitudinal
Smooth Muscleistics
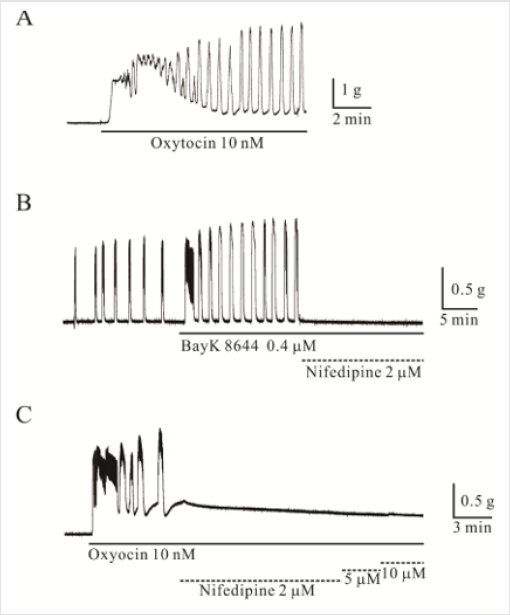
Oxytocin (OXT, 10 nM) produced tri-phasic contractions,
showing an initial contraction followed by a tonic contraction
overlapped with a phasic contraction (Figure 1). Uterine smooth
muscle produced spontaneous contractions of 1.4 ± 0.25 g with
a frequency of 0.5 ± 0.05 cycles/min (Figure 1B; n = 43). VDCCL
activator BayK 8644 enhanced the strength and the frequency
of uterine spontaneous contractions to 215 ± 49.0 % and 228
± 29.0 % of the control [n = 4 each, p < 0.05, (Figure 1B)]. These
enhanced contractions caused by BayK 8644 were completely
inhibited by nifedipine (2 μM, n = 3, p < 0.05). Phasic OXT-induced
contractions were completely blocked by 2 μM nifedipine [(Figure
1C), n = 4, p < 0.05]. The strength and frequency of OXT-induced
phasic contractions were also enhanced significantly by BayK 8644
compared to those of the control (n = 6, respectively, p < 0.05; data
not shown). These enhanced OXT-induced phasic contractions
were completely inhibited by nifedipine (2 μM, n = 6, p < 0.05, data
not shown).
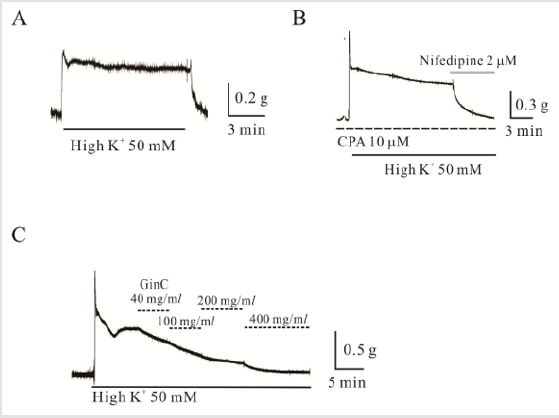
Inhibitory Effect of Ginger Extract (Gin C) on High K+-
Induced Contraction
As shown in Figure 2, high K+ (50 mM) produced tonic
contraction in uterine smooth muscle (1.8 ± 0.43 g, n=20). In the
presence of cyclopiazonic acid (CPA), high K+ (50 mM)-induced
contraction of 1.4 ± 0.75 g (n=7) and was completely blocked by
application of nifedipine [2-5 μM, n=7; (Figure 2B)]. Gin C at 40-400
mg/ mL inhibited high K+-induced contractions (Figure 2C). Gin C at
100, 200, and 400 mg/ mL inhibited high K+-induced contractions
to 46 ± 10.2%, 15 ± 4.9%, and 3.4 ± 1.4%, respectively, of the control
(p < 0.05; n = 5, data not shown). Gin C also inhibited high K+ (50
mM)-induced contraction in the presence of CPA completely (400
mg/ mL, n=5; data not shown).
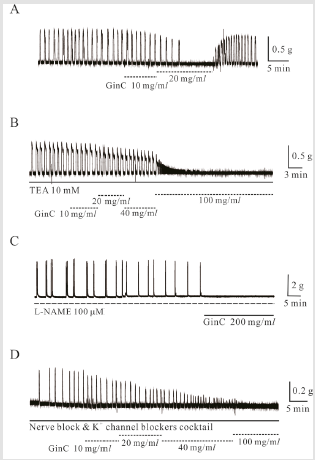
Inhibitory Effect of Ginger Extract (Gin C) on Spontaneous Contraction of Mouse Uterine Longitudinal Smooth Muscle
Spontaneous contractions of longitudinal smooth muscle were
inhibited by Gin C (10–200 mg/mL) in a reversible manner (Figure
3). Gin C at 10, 20, 100, and 200 mg/mL inhibited spontaneous
contractions to 46 ± 14.7%, 40 ± 15.5%, 26 ± 19.3 %, and 0 %, of the
control, respectively (p < 0.05; n = 5, 7, 5, and 3, respectively). Gin C
at 100 and 200 mg/mL also inhibited basal tone slightly to −0.03 ±
0.01 g and −0.05 ± 0.01 g, respectively (n = 5 and n = 3, respectively).
However, the inhibitory effect of Gin C on spontaneous contractions
was not mediated by nitric oxide (NO): Gin C at 100 mg/mL
inhibited spontaneous contractions completely in the presence of
NO synthesis inhibitor NG-nitro-L-arginine methyl ester (L-NAME,
100 μM; n = 9; Figure 3C).
Effects Of K+ Channel and Nerve Blockers on Gin
C-Induced Relaxation in Mouse Uterine Smooth Muscle
We studied the effect of Gin C on uterine smooth muscle
contractions in the presence of KBC and NBC to investigate whether
K+ channels and nerves were activated during Gin C-induced
relaxation. Gin C produced relaxation (n = 2) in the presence of
tetraethylammonium (TEA, 10 mM) which blocked Ca2+-activated
K+ (Kca) channels (Figure 3B). Gin C-induced relaxation was also
observed in the presence of KBC and NBC (Figure 3D). Gin C at 100
and 200 mg/l produced relaxation up to 14 ± 7.3 % and 0 ± 0 %,
respectively, of the control (n = 3 and n = 2, respectively).
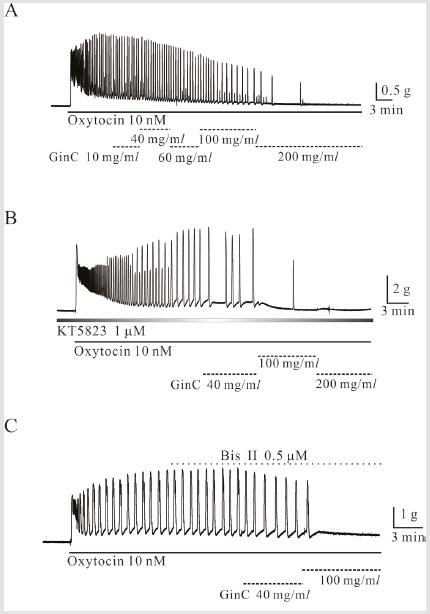
Inhibitory Effects of Gin C on Oxt- And High K+-Induced
Mouse Uterine Longitudinal Smooth Muscle Contractions
Gin C inhibited OXT-induced uterine smooth muscle
contractions. Gin C at 40, 100, 200, and 400 mg/mL inhibited OXTinduced
phasic contractions to 56 ± 12.2%, 54 ± 11.6%, 2 ± 2%, and
0% of the control, respectively (Figure 4A),( p < 0.05; n = 16, 14, 12,
and 2, respectively). In the presence of L-NAME, Gin C at 100 and
200 mg/mL also inhibited OXT-induced phasic contractions to 20 ±
7.0% and 0 %, respectively, of the control (p < 0.05; n = 10 and n =
6, respectively; data not shown). In addition, Gin C at 100, 200, and
400 mg/mL inhibited high K+-induced contractions to 46 ± 10.2%,
15 ± 4.9%, and 3.4 ± 1.4%, respectively, of the control (p < 0.05; n =
5, data not shown; Figure 4).
Inhibitory Effects of Gin C on Oxt-Induced Mouse Uterine
Longitudinal Smooth Muscle Contractions in The
Presence of Protein Kinase Inhibitors
Inhibitory effects of Gin C on OXT-induced contractions
were studied in the presence of KT 5823 and KT 5720 known to
inhibit protein kinase G (PKG) and PKA, respectively. OXT-induced
contraction was inhibited by Gin C at 40, 100 and 200 mg/l in the
presence of KT 5823 to 50 ± 20.8%, 0 %, and 0 % of the control,
respectively (n = 4, 3, and 2, respectively, p < 0.05; Figure 4C). OXTinduced
contractions in the presence of KT 5720 were inhibited by
Gin C at 20, 40, 100 and 200 mg/l to 78 ± 0.2 %, 82 ± 0.1 %, 31 ± 0.2
%, and 0 % of the control, respectively (p < 0.05, n = 5, 5, 5, and 0,
respectively; data now shown). To evaluate the involvement of PKC
in Gin C-induced inhibition of OXT-induced contractions, Gin C was
applied in the presence of a PKC inhibitor. As shown in Figure 4C, in
the presence of PKC inhibitor bisindolylmaleimie II (Bis II, 0.5 μM),
Gin C at 40 and 100 mg/l inhibited OXT-induced contractions to 36
± 20.9 % and 1 ± 0.8 % of the control, respectively (p < 0.05; n = 4
and n = 2, respectively).
Inhibitory Effects of Gin C on Prostaglandin F2α (PGF2α)-
And Acetylcholine (ACh)-Induced Mouse Uterine Smooth
Muscle Contractions
PGF2α produced tri-phasic contractions in murine uterine
smooth muscle, showing an initial contraction followed by a tonic
contraction overlapped with a phasic contraction (Figure 5). PGF2α-
induced phasic contractions were inhibited by Gin C at 100, 200,
and 400 mg/l to 39 ± 12.3 %, 20 ± 10.8 %, and 14 ± 12.0 % of the
control (n = 8, 8, and 6, respectively, p<0.05; Figure 4B). As shown
in Figure 5B, Gin C also inhibited ACh-induced phasic contractions.
In particular, Gin C at 200 mg/l completely inhibited ACh-induced
phasic contraction (n = 3; Figure 5C).
tightly linked to Ca2+ influx and Ca2+ signaling at cellular level [18].
In this study, we found that Gin C inhibited contractions of murine
uterus longitudinal smooth muscle by inhibition of VDCCL. As shown
in (Figures 1 & 2B), murine myometrial contraction was tightly
related to the activation of VDCC. Well-known pharmacological
blockers such as nifedipine inhibited myometrial contractions.
This implies that both VDCCL is important for the regulation of
myometrial contraction. In this study, Gin C inhibited high K+-,
OXT-, ACh-, and PGF2α- induced phasic contractions. Its effect was
independent of NO synthesis, protein kinases (PKA, PKG and PKC),
K+ channel, or nerve blockers (Figures 3 & 4). This finding suggests
that Gin C may inhibit mouse uterine smooth muscle contractions by
inhibiting VDCCL and/or VDCCT strongly. It has been reported that
ginger can enhance and inhibit uterine, GI tract, and airway smooth
muscle contractions by activating VDCCL [4,14,19]. In the present
study, we found that murine uterine spontaneous contractions and
OXT-induced phasic contractions were sensitive to BayK 8644 and
nifedipine (Figures 1B & 1C).
In smooth muscle, Ca2+-induced Ca2+ release (CICR) mechanisms
also known to be important to regulate smooth muscle contraction
[20-22]. As shown in Figure 2B, effect of Gin C was studied in the
presence of CPA too. Therefore, inhibition of murine myometrial by
Gin C might be responsible for the inhibition of VDCCL. However,
in fact, effects of constituents of ginger extract on smooth muscle
contractions were reported differently depending on the diverse
extract. Therefore, we will also try to do some more supplementary
experiments delicately by using other extracts from ginger in the
future. Primary dysmenorrhea may be caused by an increase in
PGF2α produced in the uterus that can hyper contract the uterine
smooth muscle and/or locally contracts blood vessels [23-24].
However, the exact mechanism of uterine spasms is currently
unclear. However, phasic contraction was also sensitive to nifedipine
in murine myometrium (unpublished data), Gin C inhibited PGF2α-
induced contractions by inhibition of VDCCL. Therefore, inhibition
of PGF2α-induced contractions by Gin C might suggests it could
reduce symptoms of dysmenorrhea.
It is well known that VDCCL performs a key role in the
regulation of smooth muscles [7-9]. However, the role of VDCCT
in such regulation is relatively unclear. In myometrium, VDCCT
in rat and human pregnant samples has been reported. In rat
pregnant myometrium, spontaneous phasic contraction has been
found to be sensitive to micromolar ranges of mibefradil [11].
Meanwhile, specific subtype of VDCCT (CaV3.1) and role of VDCCT
in the regulation of contraction in human pregnant myometrium
has been identified [25-26]. Whether the exact subtype of VDCCL
and/or VDCCT might be related to various conditions of myometrial
contractility is not fully understood yet. Furthermore, the role of
VDCCT compared to that of VDCCL in myometrial contraction is
not well studied yet. In fact, even the regulation of rat pulmonary
arterial proliferation is regulated by VDCCT through activation of
CaV3.1 channel [27]. We identified functional expression of VDCCL
(CaV1.2) in murine myometrium by performing mechanics and
immunohistochemistry (data not shown in here). However, further
study is needed to identify T-type Ca2+ channel and more functions
of it in murine and human myometria.
Ginger extracts are known to have effects on the GI tract. For
example, they can inhibit LES motility [14,19]. In these cases,
serotonergic receptors and/or cholinergic M receptors are
involved in inhibiting smooth muscle contraction [15,28]. Ginger
extract can also improve gastric emptying and IBS for gastric and
intestinal motility, respectively [12-13]. That implies ginger extract
might produce increasing and decreasing functions of smooth
muscle via affecting receptor levels too. However, we found Gin C
specifically inhibited murine myometrial contraction by inhibition
of VDCCL. Ginger plant has been used to treat inflammation,
rheumatic disorders, and diarrhea in traditional medicine [2,4,29].
Zingerone is thought to be the active antidiarrheal component
responsible for limiting endotoxin-induced diarrhea [30-31].
Therefore, some effects of ginger extracts are not direct on smooth
muscle. Meanwhile, gingerol produces dual effects (enhancing and
inhibitory) on ileal contractions in guinea-pig [12-13] through
capsaicin-sensitive neurons [32]. Shogaol- [6] from ginger can also
inhibit vascular smooth muscle proliferation by activating specific
signaling pathways [16].
A cyclooxygenase-related system in vascular smooth muscle
may be involved in regulating eicosanoid-induced contraction [16].
Additionally, gingerols exhibit various effects on the cardiovascular
system [16] while zingerone may activate the same capsaicin
receptors and/or a common pathway in trigeminal ganglion
neurons [32]. Some other Gin C components such as ginerols and/or
shogaol could also inhibit uterine contractility. Therefore, extracts
from ginger produces diverse effects on different organs via various
actions. From these results, we tried to exclude involvement of
nerves and other K+ channels by other ginger extracts [33]. As
shown in Figure 3B & 3D, the relaxing effect of ginger extract was
studied in the presence of TEA, nerve blocker cocktail. In addition,
involvement of nitric oxide (NO) in the action of antinocipective
activity by ginger extract was also reported [34]. Therefore, we
also studied and found inhibitory effects of Gin C in the presence of
and L-NAME (100 μM) on murine uterine smooth muscle (Figure
3C). Our results suggest that Gin C may produce murine uterine
relaxation by inhibiting VDCCL. This is first report showing that
inhibition of uterine smooth muscle contractility by ginger extract
obtained by dichloromethane fraction (Gin C). Our results revealed
the possibility that Gin C inhibited High K+-, OXT-, PGF2α-, and
ACh-induced uterine contraction and spontaneous contraction of
uterine longitudinal smooth muscle of mouse by inhibiting VDCCL.
The authors declare that there is no conflict of interest.
Seung Hwa Hong, Kyu Sang Kyeong, Bang Yeon Hwang equally
contributed to this work.
- Chandel KPS, Shukla G, Sharma N (1996) Biodiversity in medical and aromatic plants in India. New Delhi, India: ICAR.
- Duka JA (2002) Handbook of medicinal herbs. CRD press. Boca Raton 327-329.
- Felter HW (1992) The ecletic material media, pharmacology and therapeutics. Southwest School of Botanical Meicine. Bisbee 460-461.
- Ghayur MN, Gilani AH (2005) Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci 50(10): 1889-1897.
- Lijuan W, Kipittayanant P, Chdapngse N, Wray S, Kupittayanant S, et al. (2011) The effects of wild ginger (Costus speciosus (Koen) Smith) Rhizome extract and disogenin on rat uterine contractions. Reprod Sci 18(6): 516-524.
- Tewari, P, Prasad DN, Chaturvedi C, Das PK (1967) Preliminary studies on the uterine activity of Gloriosa superba Linn. And its adulterant Costus speciosus Sm J Res Indain Med 1: 196-202.
- Kim YC, Koh SD, Sanders KM (2002) Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol 541(3): 797-810.
- Kim YC, Sim JH, Kim YH, Kwon SC, Lee SJ, et al. (2007) Effects of polyamines on contractility of guinea-pig gastric smooth muscle. J Kor Med Sci 22(1): 48-56.
- Kim YC, Suzuki H, Xu WX, Hashitani, H, Choi W, et al. (2008) Voltage-dependent Ca Current Identified in Freshly Isolated Interstitial Cells of Cajal (ICC) of Guinea-pig Stomach. Kor J Physiol Pharmacol 12(6): 323-330.
- Lee SE, Kim DH, Kim YC, Han JH, Choi W, et al. (2014) H2 Receptor-Mediated Relaxation of Circular Smooth Muscle in Human Gastric Corpus: The Role of Nitric Oxide (NO). Korean J Physiol Pharmacol 18(5): 425-430.
- Lee SE, Ahn DS, Lee YH (2009) Role of T-type Ca Channels in the Spontaneous Phasic Contraction of Pregnant Rat Uterine Smooth Muscle. Korean J Physiol Pharmacol 13(3): 241-249.
- Banji D, Banji OJK, Pavani B, Kumar ChK, Annamalai AR, et al. (2014) Zingerone regulayes intestinal transit, attenuates behavioral and oxidative perturbations in irritable bowel disorder in rats. Phytomedicine 21(4): 423-429.
- Satoh K, Kase Y, Hayakawa T, Murata P, Ishige A, et al. (2001) Dai-kenchu-to enhances acceleratd small intestinal movement. Biol Pharm Bull 24(10): 1122-1126.
- Lohsiriwat S, Rukkiat M, Chaikomin R, Leelakusolvong S (2010) Effect of ginger on lower esophageal sphincter pressure. J Med Assoc Thai 93(3): 366-372.
- Pertz HH, Lehmann J, Roth Ehrang R, Elz S (2011) Effects of ginger constituents on the gastrointestinal tract: role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors. Planta Med 77(10): 973-978.
- Kimura I, Kimura M, Pancho LR (1989) Modulation of eicosanoid-induced contraction of mouse and rat blood vessles by gingerols. Japan J Pharmacol 50(3): 253-261.
- Pancho LR, Kimura I, Unno R, Kurono M, Kimura M, et al. (1989) Reversed effects between crude and processed ginger extracts on PGF2-induced contraction in mouse mesenteric veins. Japan J Pharmacol 50(2): 243-246.
- Shmigol AV, Eisner DA, Wray S (2001) Simultaneous measurements of changes in sarcoplasmic reticulum and cytosolic. J Physiol 531(3): 707-713.
- Ghayur MN, Gilani AH, Janssen LJ (2008) Ginger attenuates acetylcholine-induced contraction and Ca2+ signaling in murine airway smooth muscle cells. Can J Pharmacol 86(5): 264-271.
- Gravina FS, van Helden DF, Kerr KP, de Oliveira RB, Jobling P, et al. (2014) Phasic contractions of the mouse vagina and cervix at different phases of the estrus cycle and during late pregnancy. PLoS One 9: e111307.
- Kim SJ, Ahn JM, Kim YC, Park SJ, Choi JY, et al. (1998) Relationship between global cytosolic Ca2+ concentration and Ca2+-activated K+ current in rabbit cerebral arterial myocyte. J Smooth Muscle Res 34(4):159-172.
- Matthew A, Shmygol A, Wray S (2004) Ca2+ entry, efflux and release in smooth muscle.
Biol Res 37(4): 617-624.
- Hong SH, Kyeong KS, Kim CH, KimYC, Choi W, et al. (2016) Regulation of myometrial contraction by ATP-sensitive potassium (KATP) channel via activation of SUR2B and Kir 6.2 in mouse. J Vet Med Sci 78(7): 1153-1159.
- Wray S (2015) Insights from physiology into myometrial function and dysfunction. Exp Physiol 100(12):1468-1476.
- Blanks AM, Zhao ZH, Shmygol A, Bru Mercier G, Astle S, et al. (2007) Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol 581(3): 915-926.
- Monir Bishty E, Pierce SJ, Kupittayanant S, Shmygol A, Wray S, et al. (2003) The effects of metabolic inhibition on intracellular calcium and contractility of human myometrium. BJOG. 110(12): 1050-1056.
- Pluteanu F, Cribbs LL (2011) Regulation and function of Cav3.1 T-type calcium channels in IGF-I-stimulated pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 300(3): C517-C525.
- Huang QR, Iwamoto M, Aoki S, Tnaka N, Tajima K, et al. (1991) Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull 39(2): 397-399.
- Chopra A, Saluja M, Tillu G, Venugopalan A, Narsimulu G, et al. (2012) Comparable efficacy of standardized Ayurveda formulation and hydroxychloroquine sulfate (HCQS) in the treatment of rheumatoid arthritis (RA): a randomized investigator-blind controlled study. Clin Rheumatol 31(2): 259-269.
- Chen JC, Huang LJ, Wu SL, Kuo SC, Ho TY, et al, (2007) Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J Agric Food Chem 55(21): 8390-8397.
- Cho JH, Zhang S, Kim IH (2012) Effects of anti-diarrhoeal herbs on growth performance, nutrient digestibility, and meat quality in pigs. Asian-Aust. J Anim SCi 25(11): 1595-1604.
- Someya A, Horie S, Yamamoto H, Murayama T (2003) Modifications of capsicin-sensitive neurons in isolated guinea pig ileum by [6]-Gingerol and Lafutidine. J Pharmacol Sci 92(4): 359-366.
- Kyeong KS, Hong SH, Kim YC, Cho W, Myung SC, et al. (2016) Myometrial relaxation of mice via expression of two pore domain acid sensitive K (+) (TASK-2) channels. Korean J Physiol Pharmacol 20(5): 547-556.
- Khalid MH, Akhtar MN, Mohamad AS, Perimal EK, Akira A, et al. (2011) Antinociceptive effect of the essential oil of Zingiber zerumbet in mice: possible mechanisms. J Ethnopharmacol 137(1): 345-351.

 Research Article
Research Article