Abstract
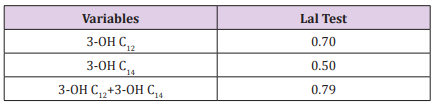
Assessment of occupational exposure to airborne endotoxins was studied for several years but still different procedures are used for sampling and analysis. Among analytical methods LAL test is the most used. A different approach is represented by chemical analyses, based on the detection of the β- hydroxy fatty acids, as markers of lipopolysaccharide (LPS). In this study an analytical method using HPLC-MS/MS was applied to quantify the content of β- hydroxy-dodecanoic (3-OH C12) and β-hydroxytetradecanoic (3-OH C14) acids in thirteen Gram- negative bacterial strains. In order to compare biological and chemical results cell suspensions were analyzed also by Kinetic Chromogenic LAL test. The results obtained by HPLC-MS/MS show that three samples did not contain 3-OH C12 at all, while 3-OH C14 was measured in a concentration range from 11.1 to 4007.5µg/L. Endotoxin concentration measured by LAL assay was in the range of 102 -103 µg/L. Pearson’s test showed that the sum of fatty acids by HPLC-MS/MS correlates positively with the LAL Test (r= 0.79) confirming that it can be considered a reliable marker of endotoxin contamination. Principal Component Analysis (PCA) confirms above results and provides additional information grouping microorganisms related with each other on the basis of their biochemical features, habitat and disease spectra.
Abbreviations: PCA: Principal Component Analysis, ODTS: Organic Dust Toxic Syndrome, OEL: Occupational Exposure Limit, LAL: Limulus Amebocyte Lysate, GC/MS: Gas Chromatography Coupled To Mass Spectrometry, HPLC-MS/MS: High Performance Liquid Chromatography Coupled To Tandem Mass Spectrometry, ATCC: American Type Culture Collection, NA: Nutrient Agar, PFW: Pyrogen Free Water, CSE: Control Standard Endotoxin
Introduction
Endotoxins are unavoidable ubiquitous microbiological contaminants; they may be present in food, environment and clinical products. Their inhalation can poses serious risks to human health, from respiratory and general symptoms such as fever, coughing, irritation and chest congestion up to chronic bronchitis and organic dust toxic syndrome (ODTS) [1,2]. For this reason, assessment of exposure to these components was studied for several years, especially in occupational environments, but a large number of different procedures are still used for sampling and analysis; this makes difficult to compare results and to establish an Occupational Exposure Limit (OEL) for these biological agents [3-5]. Among analytical methods, the most used is the Limulus Amebocyte Lysate (LAL) test, in its kinetic chromogenic version, which measures the relative reactivity of endotoxins with limulus lysate providing rapid and sensitive results. However, several critical aspects of this test are reported in literature: the cross-reactivity with other types of biomolecules such as β-d-glucans and peptidoglycans, the underestimation of the results due to a fraction non-water soluble not detectable with LAL test and the influence of endotoxin content after repeated freezing/thawing of samples [6,7]. Furthermore, this bioassay may underestimate endotoxin exposure because cellbound endotoxins, that may be associated with respiratory disease, are not detected [8].
A different approach to measure endotoxin is represented by chemical analyses, which are based on the detection of the β-hydroxy fatty acids (3-OHFAs), the major components of lipid A moiety of lipopolysaccharide, as chemical markers of LPS. The analytical methods that can be used for this purpose may be the Gas Chromatography coupled to Mass Spectrometry (GC/MS) and the High Performance Liquid Chromatography coupled to Tandem Mass Spectrometry (HPLC-MS/MS): both techniques focus on the quantification of biomarkers of endotoxins, 3-hydroxy fatty acids in the lipid A portion of LPS. Unlike bioassays, chemical analysis of 3-OHFAs allows the determination of both cellbound and non-cell-bound endotoxins, since they are chemically extracted from lipid A. Chemical methods are certainly more labor intensive and relatively expensive comparing with bioassays, but understanding the chemistry of endotoxins may help also in explaining the development of correlated diseases, and ultimately, for interventions [9]. Several methods involving GC/MS technique have been studied or developed [10,11], but comparison with the bioassay reveals relatively weak correlations: GC/ MS results are often higher than those obtained with the biological methods. In addition, GC/MS requires a long preparation time due to the need for derivatization of the sample.
HPLC-MS/MS has the advantage to be more suitable for the analysis of aqueous samples, not requiring derivatization and not exposing the analytes to high temperatures, thus avoiding the risk of degradation. Besides, the use of the tandem mass spectrometry detection increases its sensitivity and specificity. In a previous paper we presented a new analytical method by HPLC-MS/MS to quantify β- hydroxy-dodecanoic (3-OH C12, lauric acid) and β-hydroxy-tetradecanoic (3-OH C14, myristic acid) acids and a pilot study was carried out on four ATCC Gram-negative bacteria and two fungi [12]. The choice of these acids is due to the fact that previous reports indicate that shorter-chains (C10-C14) β- hydroxyfatty acids are positively correlated with endotoxin activity in the Limulus bioassay while longer-chain (C16-C18) 3-OHFAs tend to have lower or even no correlation [13]. It was also suggested that C12-C14 3-OHFAs may elicit more significant potent immunological effects in humans [14]. The main objective of this study was to evaluate the correlation between biological and chemical methods for endotoxin analysis comparing chemical results (HPLC-MS/MS) to LAL assay on thirteen Gram-negative bacterial strains of clinical and occupational interest, obtained from American Type Culture Collection (ATCC).
Materials and Methods
ATCC Gram-negative bacterial strains used in this study were: Ochrobacter anthropi-19286, Proteus mirabilis-15290, Comamonas acidovorans-15668, Pseudomonas stutzeri-53817, Flavimonas horizihabitans-35564, Acinetobacter shindleriBAA-618, Sphingomonas paucimobilis-29837, Aeromonas salmonicida-27013, Pseudomonas luteola-43330, Aeromonas hydrophila-35654, Pseudomonas aeruginosa-15442, Escherichia coli-8739 and Enterobacter aerogenes-35029, purchased from D.I.D. S.p.A, Biogenetics S.r.l. and Remel Europe Ltd. Each strain was inoculated on Nutrient Agar (NA) and incubated according to growth conditions of ATCC. Then, cell suspensions with Pyrogen Free Water (PFW) were prepared, read by spectrophotometer (OD550) and inoculated in NA to count the Colony Forming Units (final concentration ≈1,2x109 CFU/ml). Bacterial cells were mechanically lysed by vortexing at a maximum speed for 5 min with apyrogenic glass beads (0.2 mm in diameter), with intermediate cooling in ice. All samples were tested both by kinetic QCL-LAL assay and HPLC-MS/MS.
Kinetic QCL-LAL Assay
A volume of 100 µl of serial 10-fold dilutions (1, 0.1, 0.01, 0.001, 0.0001, 0.00001) of each suspension was analyzed by Kinetic QCLLAL assay (Lonza Walkersville, MD USA) and interpreted against a 5-point (concentration range from 0.005 to 50 EU/ml) standard curves of Escherichia coli (CSE). In order to obtain information about possible enhancement or inhibition reactions of the LAL assay, a replicate of each sample was spiked with a CSE standard (5 EU/ml final activity). The recovery of spiked samples was in the range of 50–200%; otherwise the measurement was repeated. Results are reported as μg/L.
HPLC-MS/MS Method
The samples were analyzed by HPLC-MS/MS technique for detection of 3-OH C12 and 3-OH C14 according to the procedure previously published [12]. Briefly, fatty acids were extracted, after alcaline hydrolysis, with n-hexane and extracts were dried in evaporator for 30 minutes at 60°C. The residue was dissolved in 0.5 ml of acetonitrile and, after filtration on 0.2μm Anotop10 IC membrane filters, was injected into the HPLC-MS/MS system. Standard solutions of 3-OH C12, 3-OH C14 acids and 3-hydroxy-tridecanoic acid (as internal standard) were prepared in acetonitrile; from these solutions, by further dilutions, the calibration and quality control (QC) samples were prepared. The HPLC device was a Perkin Elmer series 200, equipped with a Supelco Hypersyl BDSC8 (150x4.6,5μm) chromatographic column, maintained at 40°C, with mobile phase consisting of a gradient of acetonitrile and acetic acid 1% v/v in water; run time was 10 minutes at a flow rate of 1000 μl/min. The detector was an AB/Sciex API 4000 triple quadrupole mass spectrometer, equipped with a Turbo Ion Spray (TIS) probe working in the negative ion, multiple reaction monitoring (MRM) mode; the m/z ion transitions (precursor→product), corresponding to the loss of a fragment of 46 u.m.a, typical of β-hydroxy fatty acids, were monitored for both the qualitative and the quantitative analysis. The 1.4 Analyst® software was used to process the quantitative data. The method was validated in the range 10-1000 μg/L for both analytes. Results are expressed in μg/L of fatty acids.

 Research Article
Research Article