Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yuliya Kuzmenka Maskvina* and Tatiana Bich
Received: January 16, 2019; Published: January 28, 2019
*Corresponding author: Yuliya Kuzmenka Maskvina, Department of Pathology, Belarus
DOI: 10.26717/BJSTR.2019.13.002440
Abbrevations: LS: Lichen Sclerosus; SCC: Squamous Cell Carcinoma; SIL: Squamous Intraepithelial Lesion; ISSVD: International Society for The Study of Vulvovaginal Disease; WHO: World Health Organization
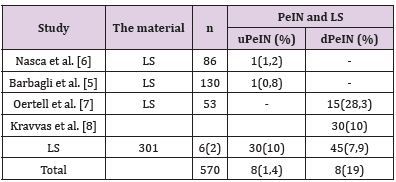
Lichen sclerosus (LS) is a chronic idiopathic skin and mucous membrane disease commonly occurring in the anogenital area of both men and women. The European Association of Urology classifies LS as a premalignant lesion occasionally associated with penile squamous cell carcinoma (SCC) [1]. The prevalence of LS in adult men according to various sources ranges from 0.0014% to 0.3% [2,3]. An important complication of the disease is the development of precancer and invasive carcinoma on its background. At the moment there are few articles in the available literature regarding the malignant potential of the LS of the penis. Almost all of those describe squamous intraepithelial lesion (SIL) associated with penile LS just near the SCC. Based on these observations, the risk of malignant transformation of LS of the penis is 2.3–8.4% [4,5]. There are few available studies investigating the relationship between male genital SL and SIL without a coexisting SCC (Table 1) [5-9]. In most of them, the authors did not set the original goal of studying this association, the latter was an accidental finding [5-7,9]. Kravvas, in their study focused on the features of clinical diagnosis and treatment of patients with male genital LS without SIL and whose having SIL without SCC on the background of this dermatosis [8]. According to published data (Table 1) male LS without co-existing carcinoma is more commonly associated with differentiated penile intraepithelial neoplasia (dPeIN) (7.9%, n=45). Vulvar intraepithelial neoplasia on the background vulvar LS without co-existing carcinoma was found being of both types in 1.2% in equal parts (uVIN 0.6% and dVIN 0.6%) [10,11]. In another study evaluating only LS cases associated with VIN without carcinoma the latter were all of usual type (HSIL) (n=27, 100%) [12].
Table 1: Squamous intraepithelial lesion (usual PeIN and differentiated PeIN) on the background of male genital LS without co-existing squamous cell carcinoma.
During recent decades there was a number of studies dedicated to the relationship of genital LS and SIL either differentiated IN or undifferentiated IN. The aim of the present study was to determine the prevalence of certain IN types in vulva and foreskin biopsies during 8 year period and to compare received data between both genders.
All penile and vulvar biopsies were retrieved from the pathology files (2012-2019) of the City Pathology Bureau in Minsk, Belarus. Slides from the original biopsy specimens of 510 (70.4%) female and 214 male patients (29.6%) were collected and revised for LS associated with IN without invasive carcinoma. The LS diagnosis was based on the presence of basal cell vacuolization, dermal homogenization and a variably dense chronic inflammatory infiltrate beneath it whether or not accompanied by epidermal atrophy or hyperplasia. SIL (high-grade SIL (HSIL) and dVIN) terminology was used according to the latest classification of the International Society for the Study of Vulvovaginal Disease (ISSVD) [13]. For penile lesions the current World Health Organization (WHO) classification with differentiated penile IN (dPeIN) and undifferentiated PeIN (uPeIN) was applied [14].
LS was found in 48.6% (n=248) of vulvar and in 43.5% (n=93) of penile species. The meanage for women was 62±9,7 years (26- 89) and 46±13,6 (19-88) years for men. In the series of vulvar LS VIN was observed in 5 cases (2%), 60% of which (n=3) were classified as HSIL and 40% (n=2) as dVIN. The median age of LS female patients with HSIL and dVIN was 77 (55, 77, 80) and 79 years (76, 81) respectively. In these 93 penile species with LS PeIN lesions were diagnosed in 8 cases (10.8%), among which uPeIN was observed in 50% (n=4) as was dPeIN (n=4). The median age of LS male patients with uPeIN was 32 years (32, 32, 32, 73) and dPeIN was 44.5 years (29, 44, 45, 66).
According to our data intraepithelial neoplasia related to LS without co-existing SCC in both genders is likely to be of differentiated and undifferentiated types in equal parts. The risk of LS progression to PeIN is 10.8% that is 5.4 times higher in comparison to VIN (p=0.0047). Women suffering from vulvar LS in association with VIN with no background SCC are 2 times older than in men (median age for women is 77 years, for men - 38 years) (p=0.005).


