Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Xiaoguang Wang1, Xiaodan Yang1, Fei Chen1, Wei Chen*2, and Zheng xiang Zhong*1
Received: September 18, 2018; Published: October 03, 2018
*Corresponding author: Zheng-Xiang Zhong, The Second Affiliated Hospital of Jiaxing University, China Wei Chen, Zhejiang Academy of Traditional Chinese Medicine, China
DOI: 10.26717/BJSTR.2018.09.001815
Vitamin D Receptor (VDR) polymorphisms have been investigated in the context of some autoimmune liver diseases, such as primary biliary cirrhosis (PBC) and autoimmune hepatitis (AIH). However, the association between VDR and autoimmune live diseases is still controversial and ambiguous. Hence, we integrate previous finding and explore whether this polymorphism is associated with susceptibility to autoimmune liver diseases. A meta-analysis was performed by Pubmed, Ovid, Medline, and Web of science databases, with the last report up to February 2012. Casecontrol studies containing available genotype frequencies of VDR were chose. The odds ratio (OR) and its 95% confidence interval (95%CI) were used to assess the strength of association. A total of 6 publications containing 9 studies (7 studies about VDR, 2 studies about AIH) including 844 cases and 1,522 controls were identified. The combined results based on all studies showed that there was a statistically significant link between Apa1 and autoimmune liver diseases (OR=0.85, 95%CI 0.74-0.96, P=0.058, for a vs. A; OR=0.75, 95%CI 0.58-0.97, P=0.212, for aa vs. AA; OR=0.78, 95%CI 0.63-0.98 P=0.235, for Aa vs. AA; OR=0.77, 95%CI 0.63-0.94, P=0.231, for Aa+aa vs. AA), while the Bsm1 and Taq1 don’t show the association with autoimmune liver diseases. The current meta-analysis shows that based on current evidence from published studies Apa1 may be a lowpenetrant risk factor for autoimmune liver diseases.
Abbreviations: VDR (Apa1, Bsm1, Fok1, Taq1) Polymorphism; Autoimmune Liver Diseases; Primary Biliary Cirrhosis (PBC); Autoimmune Hepatitis (AIH); Meta-Analysis
Primary biliary cirrhosis (PBC) is a chronic and progressive cholestatic liver disease, eventually resulting in liver fibrosis, and is a liver-specific autoimmune disease [1,2]. Although the etiology of PBC still remains largely unknown, accumulating evidence strongly suggests the involvement of both genetic and environment components in susceptibility as well as progression of PBC [3,4]. Indeed, genetic factors are known to contribute to PBC pathogenesis.
Autoimmune hepatitis (AIH) is an immune-mediated chronic inflammation of the liver of unknown etiology which is characterized by elevated serum transaminase levels, hypergammaglobulinemia, serum autoantibodies, histologic signs of interface hepatitis, and a response to immunosuppressive treatment [5,6]. Although its etiology is unknown, genetic factors have been implicated in the pathogenesis. A number of genes outside the MHC locus may play a role in susceptibility to autoimmune liver diseases. The vitamin D receptor (VDR) is a candidate. The VDR is an intracellular receptor belonging to the steroid nuclear family. It is the specific receptor for vitamin D, an immunomodulator which vitamin D exerts its effects through. Certain polymorphisms of the VDR gene may modify vitamin D function. It is found that over 30 polymorphisms within the VDR gene [7]. Only a limited number of these polymorphisms have been studied in relation to autoimmune diseases [8-10]. Four common polymorphisms studied in autoimmune liver diseases are ApaI (rs7975232), BsmI(rs1544410) FokI(rs10735810) and TaqI(rs731-236) [10], however, the results were inconclusive and inconsistent [10-15]. To help get a decisively conclude, we join pieces of evidence from published literature for a meta-analysis. To our knowledge, no meta-analysis regarding this issue has been previously reported.
In the present study, we searched the eligible studies using the keywords “VDR”, “Apa1”, “Bsm1”, “Fok1”, “Taq1”, “polymorphism’’ or ‘‘variation’’, and “PBC” or “AIH” in Pubmed, Ovid, Medline, and Web of science databases, with the last report up to February 2012. We also used the PubMed option ‘‘related articles’’ in each research article to search potentially relevant articles. Studies published in any language were included in our meta-analysis if they met the criteria:
a) the studies were a case- control study;
b) the association is between “VDR” gene including “Apa1”, “Bsm1”, “Fok1”, “Taq1” polymorphism and “PBC” or “AIH”;
c) the data is sufficient to estimate an odds ratio (OR) with its 95% confidence interval(95%CI). When multiple publications reported on the same or overlapping data, we chose the largest or most recent publication, as recommended by Little et al. [16].
Major exclusion criteria were:
i. no control population,
ii. no available genotype frequency,
iii. study with inconsistent data. Each article was checked independently by two investigators.
The following variables were extracted from each study: the first author, year of publication, country, ethnicity, number of cases and controls, and numbers of cases and controls in different genotype distribution, respectively. Information from all eligible publications was carefully reviewed and extracted, according to the inclusion and exclusion criteria listed above, independently by two of authors. Disagreement was resolved by discussion between the two authors. If they could not reach an agreement, another investigator adjudicated over the disagreement
The quality of these studies was assessed according to the confirmation of Hard- Weinberg equilibrium (HWE) for the genotype distribution of VDR (Apa1, Bsm1, Fok1, Taq1) polymorphism in the controls [17]. Studies with the genotype distribution of VDR polymorphism in controls in agreement with HWE (P>0.01) were defined as high quality studies. On the opposite, studies with departures from HWE in the controls were defined as low quality studies.
Pooled OR together with 95%CI were calculated by a fixed effects model or a random effects model according to the heterogeneity. The statistical heterogeneity among studies was checked by the Chi square-based Q test [18]. When the heterogeneity was absent (P>0.05), the fixed-effects model (the Mantel–Haenszel method) was used to estimate the pooled OR [19], otherwise, the randomeffects model (the Der Simonian and Laird method) was used [20]. Five different ORs were calculated:
a) The allele contrast model (a vs. A),
b) The homozygote codominant model (aa vs. AA),
c) The heterozygote codominant model (Aa vs. AA),
d) The dominant model (Aa+ aa vs. AA), and
e) The recessive model (aa vs AA+ Aa) [21].
The potential publication bias was tested using Egger’s test (P<0.05 considered to be representative of a statistical significance) [22]. Besides, we also tested influence analysis by omitting each study to find potential outliers. All the statistical analyses were performed using STATA version 12.0 (Stata Corp, College Station, TX).
There were 6 publications containing 9 studies including 844 cases and 1,522 controls were identified according to the inclusion and exclusion criteria listed above. Six publications including 7 studies is about PBC [10-15], and 2 studies is about AIH [10,13]. A database, including first author, published year, original country, ethnicity, type, polymorphism, sample size of cases and controls was established according to the extracted information (Table 1). Genotype and allele distributions for each study about Apa1 polymorphism association PBC or AIH is shown in Table 2, Bsm1 is shown in Table 3, and Taq1 is shown in Table 4. In the publication Vogel et al. [10], and Fan et al. [13], the OR was presented separately according to PBC study and AIH study, and each study was considered separately for this meta-analysis. And in the study of Tanaka et al. [14], the OR was separately divided into Japanese and Italians study, and each study was chose separately into this meta-analysis too. Meanwhile, HWE for each study about Apa1, Bsm1 and Taq1 are shown separately in Tables 2-4. All studies were confirmed HWE (P>0.01) except 4 studies (one study for Apa1, 1 study for Bsm1, and 2 studies for Taq1), and according to the quality assessment criteria, the 4 studies were considered to low quality studies.
Table 2: The relationship between the ApaI polymorphism in the vitamin D receptor gene and PBC or AIH HWE in controls a allele frequency of controls.
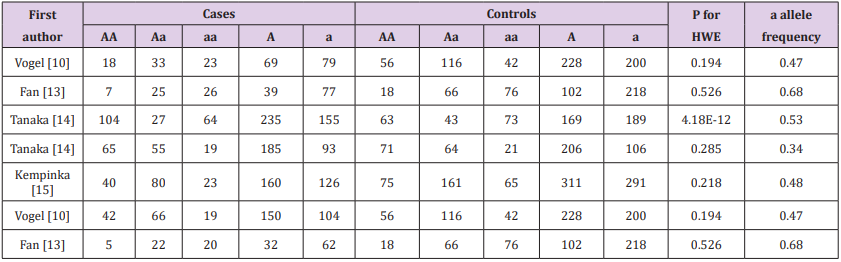
Table 3: The relationship between the BsmI polymorphism in the vitamin D receptor gene and PBC or AIH HWE in controls b allele frequency of controls
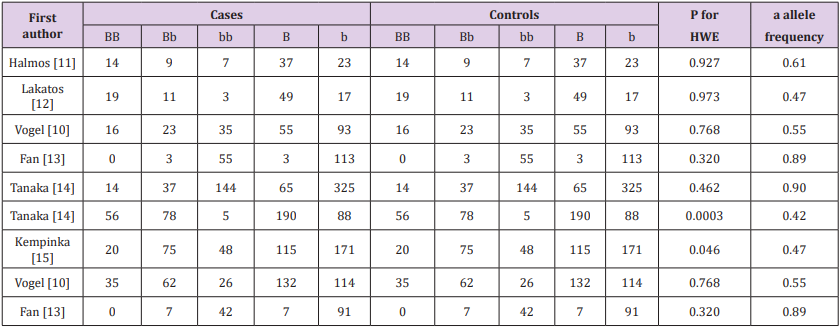
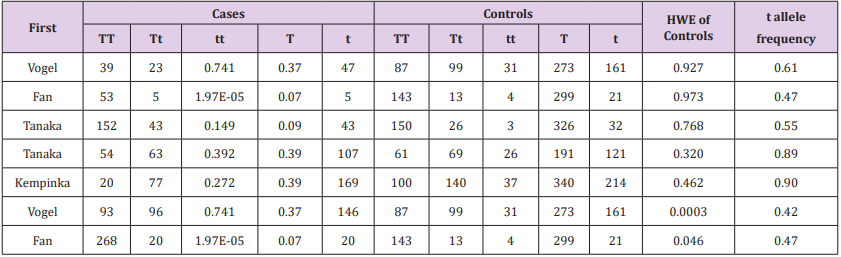
Table 4: The relation between the Taq1 polymorphism in the vitamin D receptor gene and PBC or AIH HWE in controls. an allele frequency of controls.
The results of the association between the Apa1, Bsm1 and Taq1 polymorphisms and the risk of autoimmune liver diseases, and the heterogeneity test were shown in Table 5.
Table 5: Summary ORs and 95% CI for various contrasts in VDR polymorphisms P value for heterogeneity, when p<0.05 Estimates for random effects.
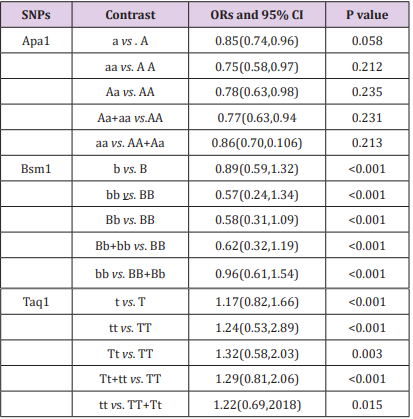
A total of 783 autoimmune liver diseases (609 cases for PBC and 174 cases for AIH) and 1,384 controls (1,010 controls for PBC and 374 controls for AIH) were included in the meta-analysis on the relationship between the Apa1 polymorphism and the risk of autoimmune liver diseases (Table 2). The results showed a significant association between Apa1 polymorphism with autoimmune liver diseases: (1) the allele contrast model (a vs. A), (OR=0.85, 95%CI 0.74-0.96, P=0.058 for heterogeneity), Figure 1; (2) the homozygote codominant model (aa vs. AA) (OR=0.75, 95%CI 0.58-0.97, P=0.212 for heterogeneity), Figure 2; (3) the heterozygote codominant model (Aa vs. AA), (OR=0.78, 95%CI 0.63-0.98, P=0.235 for heterogeneity), Figure 3; (4) the dominant model (Aa+ aa vs. AA), (OR=0.77, 95%CI 0.63-0.94, P=0.231 for heterogeneity), Figure 4.
Figure 1: Forest plot for the association between the Apa1 polymorphism and the risk of autoimmune liver diseases (for a vs. An allele contrast model). The study is shown by the point estimate of the OR (the size of the square is proportional to the weight of each study) and 95 % CI for the OR (horizontal lines); the pooled OR and 95 % CI have been appropriately derived from the fixed effects model.
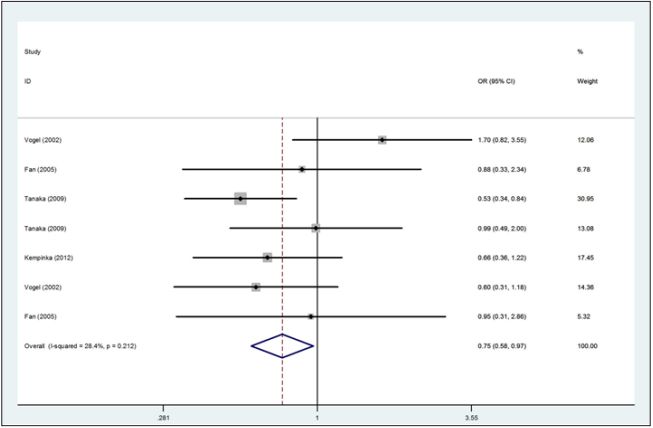
Figure 2: Forest plot for the association between the Apa1 polymorphism and the risk of autoimmune liver disease (for aa versus AA homozygote codominant model, fixed effects model).
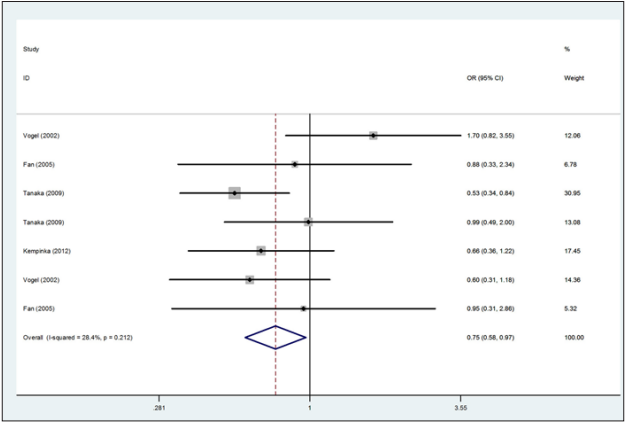
Figure 3: Forest plot for the association between the Apa1 polymorphism and the risk of autoimmune liver disease (for Aa versus AA heterozygote codominant model, fixed effects model).
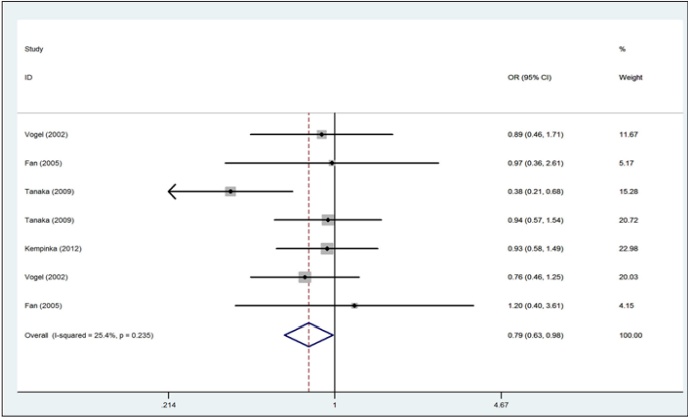
Figure 4: Forest plot for the association between the Apa1 polymorphism and the risk of autoimmune liver disease (for Aa+ aa versus AA dominant model, fixed effects model).
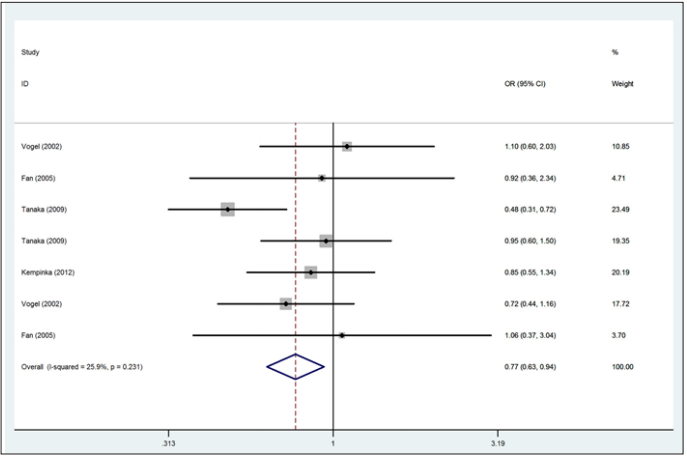
A total of 844 autoimmune liver diseases (672 cases for PBC and 172 cases for AIH) and 1,522 controls (1,148 controls for PBC and 374controls for AIH) were included in the meta-analysis on the relationship between the Bsm1 polymorphism and the risk of autoimmune liver diseases (Table 3). The results showed no significant association between the allele contrast (b vs. B) with autoimmune liver diseases (OR=0.89, 95%CI 0.59-1.32, P<0.001 for heterogeneity). We also did not find the significant association in the recessive and dominant models (Table 5).
A total of 776 autoimmune liver diseases (609 cases for PBC and 167 cases for AIH) and 1,366 controls (989 controls for PBC and 377 controls for AIH) were included in the meta-analysis on the relationship between the Bsm1 polymorphism and the risk of autoimmune liver diseases (Table 4). We did not find any significant association between Taq1 polymorphism with autoimmune liver diseases (Table 5).
As 2 publications including 4 studies (two for PBC, 2 for AIH), a meta-analysis cannot been processed, but Vogel et al. [10] thought homozygote codominant “ff” was a protect factor (OR=0.50, 95%CI 0.28-0.92, P=0.02) and Fan et al. [13] regret as high-risk factor (OR= 2.18,95% CI 1.07–4.43, P=0.019) for the risk of AIH.
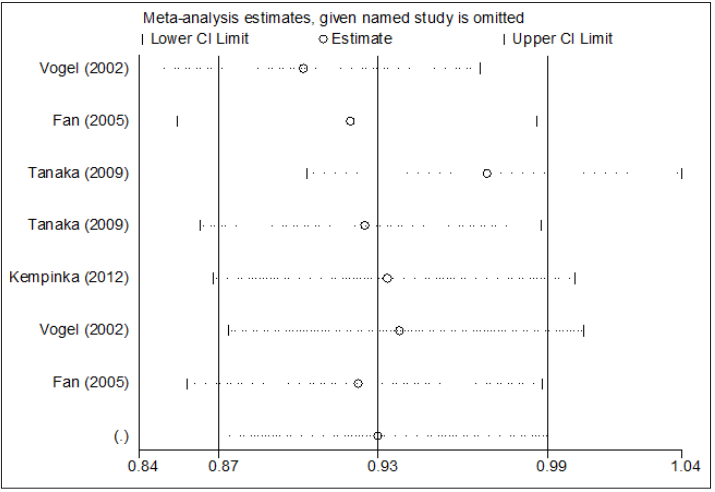
According to each study may influence the overall pooled OR, we processed influence analyses by deleted one single study from the overall pooled analysis each time, to check the influence of the removed data set to the overall ORs. Sensitivity analysis indicates that no individual study significantly affects the pooled OR (Figure 5).
Figure 5: Influence analysis for a versus A in the overall meta-analysis. This figure shows the influence of individual studies on the summary OR. The middle vertical axis indicates the overall OR and the two vertical axes indicate the pooled OR when the left study is omitted in this meta-analysis.The two ends of the dotted lines represent the 95 % CI.
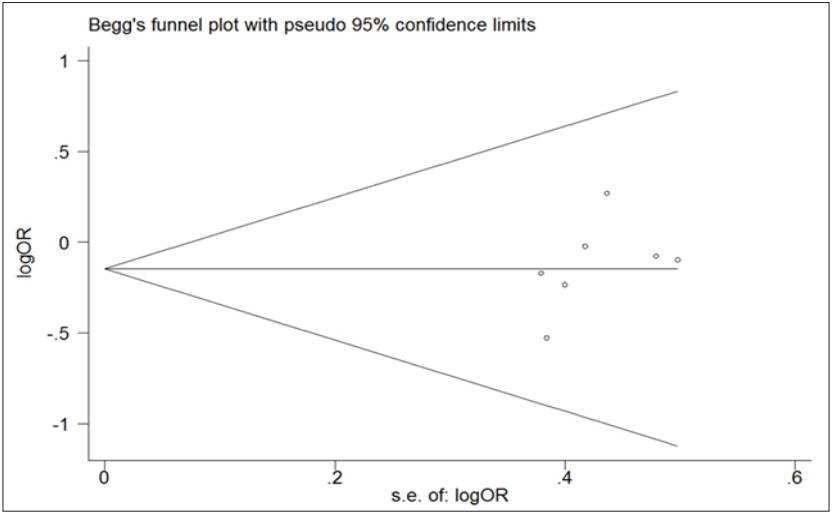
Both Begger’s funnel plot and Egger’s test were conducted to estimate the publication bias of articles. And the shape of funnel plots did not reveal apparent symmetry. But still no statistical significant evidence of publication bias was found in any comparison models. Then, Egger’s test was used to provide statistical evidence for funnel plot symmetry, we obtained the result (t =1. 26, P=0.264 for a vs. A Figure 6). These findings suggest that publication bias was absent. For Bsm1 and Taq1, there was also no publication bias present (data not shown).
Figure 6: Begg’s funnel plot of publication bias test for the Apa1 polymorphism (a vs.A). Each point represents a separate study for the indicated association. The vertical axis represents log [OR] and the horizontal axis means the standard error of log [OR]. Horizontal line and sloping lines in funnel plot represent random-effect summary OR and expected 95 % CI for a given standard error, respectively. Area of each circle represents the contribution of each study to be pooled OR.
Vitamin D receptor (VDR) gene plays an important role in the Vitamin D pathway and belongs to the steroid hormone family of nuclear reports which are responsible for the transcriptional regulation of several hormone responsive genes. It is found to be expressed by human immune cells including macrophages, dendritic cells, and T and B lymphocytes more than 25 years ago [23]. VDR is ligated with vitamin forming the vitamin D/ VDR complex, which are translocated to the nucleus, and form a heterodimer with the transcription factor IIB (TFIIB) to a vitamin D response element (VDRE) and leads to transcriptional suppression or activation of Vitamin D response genes [24]. Abnormal function of VDR, which is attributable to the VDR gene polymorphisms and altered transactivation, may affect immune cells interaction with vitamin D. Prior studies of VDR polymorphisms and autoimmune liver diseases risk had limited in sample size and yielded into inconsistent results.
We performed the meta-analysis to analyze the association between autoimmune liver diseases risk and VDR (Apa1, Bsm1 and Taq1) polymorphisms in the gene and the results indicates that Apa1 is associated with autoimmune liver disease risk, while Bsm1 and Taq1 polymorphisms don’t show the association. These results might help to explain individual differences in susceptibility to autoimmune liver diseases. When it comes to Apa1, 4 studies found no association with autoimmune liver diseases and Vogel et al. [10] think “tt” is a high pathogenic factor (OR=1.85, 95%CI 1.02-3.35, P=0.04) for PBC. But Tanaka et al. [14] find “AA” is a high pathogenic factor (OR=2.01, 95%CI 1.39-3.19, P=0.001) for PBC in Japanese. And our results confirmed the “t” allele is a low-penetrant risk factor for autoimmune liver diseases.
At the same time, our meta-analysis did not find any association of the other SNPs (Bsm1 and Taq1) alone was associated with autoimmune liver diseases. A few studies have generated some relevant data, but their findings had been contradictory. 9 studies assessed Bsm1 associated with an autoimmune liver diseases [10- 15]. Two studies [10,13] reported Bsm1 was uncorrelated with AIH, 3 studies [10,13,15] thought “bb” was a high pathogenic factor for PBC, and 4 studies [11,12,14] got a contrary conclusion. For the Taq1, 4 studies [13,14] demonstrated that Taq1 polymorphisms didn’t affect the risk of autoimmune liver diseases, 3 studies [10,15] thought Taq1 is a low-penetrant risk factor for the risk of autoimmune liver diseases. Study showed the Apa1, Bsm1 and Taq1 polymorphisms were in strong linkage disequilibrium [25]. But according the previous studies the association between the common Bsm1/Apa1/Taq1 haplotypes and VDR function is inconsistent [26-28]. Because we could not gain more detailed individual information on genotypes of the three polymorphisms in this study, we could not process the pooled analysis of linkage disequilibrium. More studies should be performed in future to uncover the pooled analysis of linkage disequilibrium.
The main purpose of performing the meta-analysis is to improve statistical power and obtain more compelling results by increasing the sample size. However, this study had some limitations. First, the subjects of the study were also small, and the peoples are mixed. Second, the controls were not uniformly defined. Third, our study was based on unadjusted published estimates, and for this reason we were unable to adjust them by possible confounders such as age, sun exposure, dietary vitamin D intake. In spite of these, our meta-analysis also had some advantages. First, the quality of case– control studies included in this meta- analysis was satisfactory and met our inclusion criterion. Second, we did not detect any publication bias indicating that the whole pooled result should be unbiased. In summary, this meta-analysis provided evidence of the association between VDR (Apa1, Bsm1, and Taq1) polymorphism and the risk of autoimmune liver diseases,and indicated that, based on current evidence from published studies, Apa1 may be a low-penetrant risk factor for autoimmune liver disease. Further replication studies in distinct populations will be necessary to confirm the ethnic stratification of the association. And more large well-designed studies should be on the association between Fok1 with autoimmune liver diseases.
-This work was supported by grants from the Hospital Administration Center of Wuxi, China (No. YGZ1016, YGZ1106).


