Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Farid S Nassar*
Received: February 01, 2018; Published: February 12, 2018
Corresponding author: Farid S Nassar, Department of Animal Production, Faculty of Agriculture, Cairo University, Giza 12613, Egypt
DOI: 10.26717/BJSTR.2018.02.000751
Poultry has become a great important for producing meat and egg to face increasing in the world population size form year to the next. In recent years, poultry were used as an experimental animal model in medical research and pharmaceutical industry because of its advantages. The current review will indicate the different application of poultry and its products in wide application in biotechnology in medical research and pharmaceutical industry to study and treatment human diseases. Also, some of poultry diseases viruses used for treatment of human diseases. Using of poultry and chick embryos in medical research and pharmaceutical industry will witness great developments according to the latest knowledge in cell and animal transgenesis in the near future.
Keywords: Biotechnology; Experimental animal model; Human diseases; Medical research; Poultry
Abbreviations: CAV: Chicken Anemia Virus; IBDV: Infectious Bursal Disease Virus; NAFLD: Nonalcoholic Fatty Liver Disease; WC-As: White Carneau; IBDV: Infectious Bursal Disease Virus; HCV: Hepatitis C Virus; SPF: Specific Pathogen-Free
During the past few years there has been a notable increase in the demand for poultry meat and egg production due to its low cost, good nutritional profile and suitability for further processing. Moreover, current forecasts and projection studies have predicted that the expansion of the poultry market will continue in the future. This growing demand has led to progressive improvements in genetic selection to produce fast-growing birds [1-3] and increase egg production from egg type chicken [4]. On the other hand, in recent years poultry were used not only as experimental animal model in pharmaceutical and medical research but also in in the pharmaceutical industry because of its advantages such as their short breeding time, simple management, and high productivity are of interest for recombinant therapeutic production in their eggs [5]. Also, Chick embryos are a significant historical research model in basic and applied sciences. Poultry eggs and cell lines derived from embryonated eggs have found wide application in industry and biotechnology. Avian derived cell lines are an alternative for virus production because they provide a simple, flexible system that can be controlled for an extended time with the capability for rapid vaccine production during a pandemic [5]. It is also strange to use viruses that infect poultry a pathogen causing economic losses in chickens in the treatment of human disease such as Infectious bursal disease virus (IBDV) [6] and the chicken anemia virus (CAV) [7]. Also, many human diseases were studded by using poultry such as cancer, nonalcoholic fatty liver disease (NAFLD), Atherosclerosis [8-11]. Recent advances originated by using transgenic approach highlight the use of avians as animal models in the medical research and pharmaceutical industry.
Atherosclerosis is one of the leading causes of death in human. As a prevalent complication of type II diabetes, the disease is affecting an increasing population in economically developed countries. Understanding the mechanisms underlying specific pathologies and diseases in human depend on animal models that match specific human pathologies. Atherosclerosis is one of the considerable human diseases that depend on models predominately at the earliest stages of the disease. Pigeon is one of several good animal models for atherosclerosis [12-13]. White Carneau (WC- As) pigeons are susceptible to spontaneous atherosclerosis at the celiac bifurcation of the aorta, whereas show Racer (SR-Ar) pigeon is resistant to the development of atherosclerosis under identical diet and housing conditions, and with similar blood cholesterol levels [14].In addition, many research indicated gene expression which may associated with Atherosclerosis disease development, progression, or both [8-14].
On the other hand, the reproductive strategy for avian species that produce a sequence of eggs is dependent upon the maintenance of a small cohort of viable, prehierarchal follicles. It is from this cohort that a single follicle is selected on an approximate daily basis to initiate rapid growth and final differentiation before ovulation [15]. Commercial laying hens spontaneously develop ovarian cancer at a high rate, and susceptibility to this disease has been associated with ovulatory events in women. Also, ovulation or events associated with ovulation increases the prevalence of ovarian cancer in hens. In addition, Genetic selection for high productivity in commercial laying hens has generated an efficient and valuable food source as well as an important animal model for human ovarian cancer which is the fifth leading cause of death from all cancers among women and is the leading cause of death from gynecological malignancies [11,16].
Infectious bursal disease virus (IBDV) is a highly infectious virus with a bisegmented double-stranded RNA (dsRNA) genome which causes immunosuppression in chickens. Infectious bursal disease virus has been used as a therapeutic agent without any toxicity in clinical trials with patients suffering from acute and chronic hepatitis C virus (HCV) infections[17,18]. Also, IBDV was used as a vector which shows efficient expression when carrying Hepatitis C Virus Epitopes and demonstrates the potential of IBDV as a vector [6]. The chicken anemia virus (CAV) was first described in 1979 in commercially produced chickens [19]. CAV can lead to great economic loss during intensive chicken farming and control of the virus through vaccination is currently standard practice in poultry farm. The chicken anemia virus (CAV) protein apoptin is known to induce tumor cell-specific death when expressed Apoptin has attracted considerable interest due to its ability to mediate cell death selectively in cells that have undergone an oncogenic transformation. Chicken anemia virus (CAV) is a single-stranded circular DNA virus that carries 3 genes, the most studied of which the gene is encoding VP3, also known as apoptin. This protein has been demonstrated to specifically kill transformed cells while leaving normal cells unharmed. In addition, apoptin is a sensor of DNA damage signaling which induces it to migrate to the nucleus during viral replication. Thus, apoptin has very important advantages in therapeutic different types of human cancer [7,20].
Oxidative stress and DNA damage play critical roles in various diseases and pathological conditions in human. Moreover, cancer is the second leading cause of human mortality at global level, and commercial and scientific sectors show very strong interests in discovering new anticancer agents from natural sources [21]. In recent years, some scientific arguments indicated that certain bioactive peptides and proteins could have several beneficial effects on human health [22]. Egg is an important source for minerals, vitamins, lipids, and proteins which also considered as an excellent source for biologically active substances [23]. For example, egg yolk is containing Phosvitin which is a phosphoglycoprotein present represents about 7% of yolk proteins [24]. Phosvitin has a high iron-binding capacity, and the best physicochemical conditions for the high iron-binding capacity are pH 6.5 and the ionic strength of 0.15M [25]. Phosvitin also exhibited a strong antibacterial activity against a broad spectrum of bacteria and has anti-tyrosinase and melanin biosynthesis activities [26]. Moreover, the Phosvitin showed protective effects against the oxidative stress-induced DNA damages in human leukocytes which suggested using Phosvitin as an anticancer agent for humans [27]. In addition, ovotransferrin and its enzyme hydrolysates produced in eggs can be used as natural growth inhibitors of human cancer cell lines [28].
Table 1: A number of recombinant proteins produced in avian eggs [5].
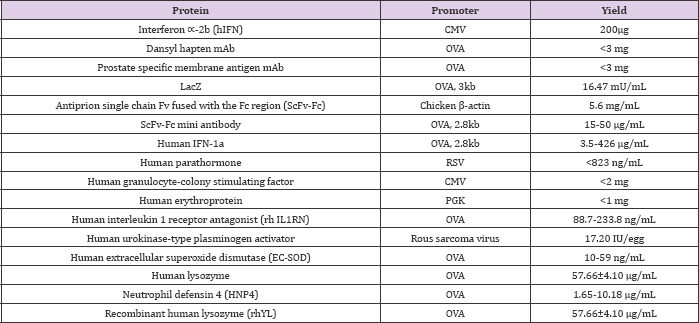
Over few decades, specific pathogen-free (SPF) fertilized poultry eggs have been considered permissive hosts for different t types of viral strains to produce mammalian vaccines [29,30]. f However, industrial sector virus production requires a large amount of SPF fertilized eggs [30]. Avian derived cell lines are an alternative for virus production because they provide a simple, pliable system that can be controlled for prolonged time with the ability for rapid vaccine production during a pandemic [31]. In ; addition, chicken embryo-derived cells, as primary cell substrates, have been evaluated as hosts for viruses that maintain genomic stability after repeated cell passages [31]. In addition, collectively, these numerous benefits make avian eggs one of the best sources for production of recombinant proteins (Table 1) and using avian cell lines for vaccine production (Table 2). Using poultry in industry and research has gain attention in the field of avian transgenesis according to it's the tremendous potentials [32]. Transgenesis in avians allows producing recombinant protein production, such as therapeutic monoclonal antibodies. Moreover, Transgenesis in avians allows for the possibility of recombinant protein production, such as therapeutic monoclonal antibodies [33].
Table 2: Commercialized avian cell lines for vaccine production [5].
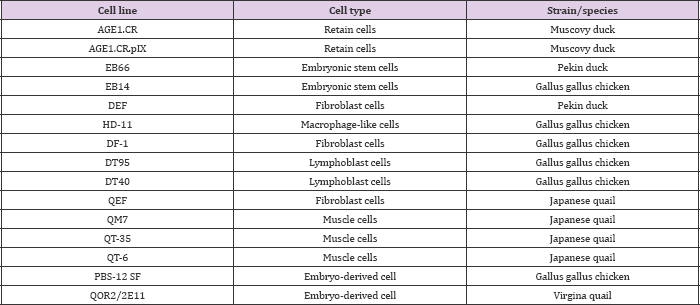
Avian species introduced potential advantages for use in medical research and pharmaceutical industry. Recently, tremendous effort has focused on avian disease viruses, avian eggs, and related cell lines for germline modification in order to create an excellence system platform for the production of therapeutic antibodies, vaccine manufacturing, and recombinant proteins. The application of using poultry and chick embryos in medical research and pharmaceutical industry will witness great developments according to the latest knowledge in cell and animal transgenesis in the near future.


