Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jordyn Vienneau*, Jay Bauman, Sandro Nigg, Benno M Nigg and Scott E Jarvis
Received: January 17, 2018; Published: January 24, 2018
*Corresponding author: Jordyn Vienneau, Senior Biomechanics Technician, Human Performance laboratory, KNB 218, Faculty of Kinesiology, University of Calgary, 2500 University Drive NW, Calgary, AB, Canada, 2N1N4, (403) 220-5051, Canada, USA
DOI: 10.26717/BJSTR.2018.02.000689
The objective of this study was to investigate eight week training effects, followed by eight weeks of washout effects with a newly developed medical device (the SurroGait Rx™ [Orpyx Medical Technologies Inc., Calgary, Canada]) on physical and psychological impacts of multiple sclerosis (MS), gait, and balance parameters in individuals with MS. Seven individuals with MS completed the 16-week protocol, which included baseline, post-training (8 weeks) and post-washout (16 weeks) sessions. During each session, impact of MS on the patient (MSIS-29), gait (T25FW, stride variables), and balance (postural sway trajectory) parameters were assessed. Results showed significant improvements in the MSIS-29 scores after training, no effect on gait variables, and longer postural sway trajectories with the device on versus off. There were no significant training effects in the balance tasks. Future studies are warranted using individuals with more advanced cases of MS to more fully understand the potential benefits of the SurroGait Rx.
Keywords: Sensory substitution; Quality of life; Peripheral neuropathy; Rehabilitation; Balance; Inertial measurement units
Abbreviations: SSS: Sensory substitution systems; MS: Multiple sclerosis; MRC: Manual Muscle Testing; MNSI: Michigan Neuropathy Screening Instrument; NDS: Neuropathy Disability Score
Sensory substitution systems (SSS) are a class of medical technologies that seek to compensate for compromised sensory organs [1]. SSSs are theorized to leverage the phenomenon of Neuroplasticity, such that the artificial sensory feedback is translated to an interpretable signal by the user. Neuroplasticity refers to the capacity of the central nervous system to rewire itself to accomplish new behavioural patterns or neural processes [2]. The most successful implementations of sensory substitution have been to transform visual information into tactile or auditory stimuli in blind persons, but SSSs have also been demonstrated to convey vestibular and even non-physiological information, such as compass orientation [3-9].
Multiple sclerosis (MS) is a neurological condition that frequently causes impaired plantar sensation, thus leading to impairments in balance and walking [10-12]. It has been reported that individuals with MS have slower walking speeds with shorter stride lengths, higher cadence, and larger variability than healthy controls [13,14]. While standing, balance deficits have also been reported in MS patients, especially for narrow or tandem stance postures, which are more challenging [15-18]. Because of these common impairments, and limited conventional treatments, a number of new and innovative training strategies have been developed with the aim of improving MS patient mobility, and thus quality of life. For example, studies have been conducted with robot-assisted treadmill walking [19-21], virtual reality glasses [22], rhythmic auditory stimuli [23,24], Tai-Chi [25,26], and physiotherapy [27,28], all of which yielded positive effects on gait and balance parameters.
A new medical device called the SurroGait Rx™ (Orpyx Medical Technologies Inc., Calgary, Canada; (Figure 1) has been developed with the goal of compensating for lost sensory function in the feet. The system includes a pair of shoe insoles with pressure sensors with wireless transmitters, and two vibrotactile arrays held against the back using a custom vest. Each sensor insert has eight discrete sensing elements positioned to correspond to the bony prominences of the foot. A shoe pod, worn on the top of the shoe, measures each sensor at a frequency of 100 Hz, and transmits the pressure data to the corresponding vibrotactile array. Measurements are transmitted using a low-latency wireless protocol to ensure there is no lag between sensor and motor activation. The latency between sensor measurement and corresponding motor activation is approximately 10 ms. each vest contains two arrays of eight small vibration motors.
Figure 1: Illustration of SurroGait Rx device. Patients wore a vest that housed 16 vibrotactile motors in the back (eight per side). The physical location of the vest motors corresponded directly to the location of the eight sensors located in each insole, worn in the patient’s shoes. Activation of the insole sensors (pressure) resulted in vibration of the corresponding motor felt on the back via Bluetooth TM technology.
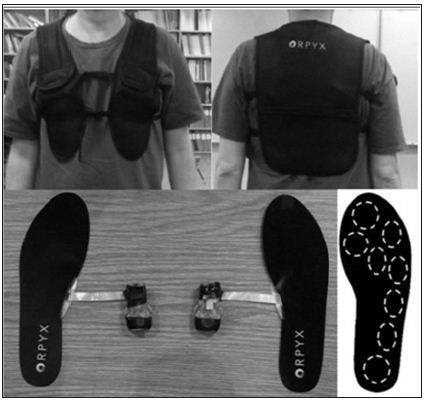
The arrays are positioned side by side in the vest, where the vibrotactile array one the left side of the vest corresponds to the left foot and vice-versa. As the user walks, the sensor-embedded insoles provide real time plantar pressure feedback to the vibrotactile motors. The feedback is given both with respect to the physical location of the pressure on the plantar surface of the foot, which is relayed to corresponding locations on the back, and magnitude of the pressure, which is relayed via the intensity of the vibration felt on the back. The substitute sensory information that this device provides intends to subsequently improve the patient’s motor control of gait and posture via input into central nervous system afferent sensory pathways. In a healthy individual (Figure 2A), proprioceptive information arrives via large myelinated Absensory nerve fibres In the dorsal column of the lumbar spinal cord. Nerves within this tract ascend through the tractus gracilis to the level of the medulla where they synapse in the nucleus gracilis. After synapsing, neurons travel through the medial lemniscus to the ventral posterolateral nucleus of the thalamus finally, neurons project from the thalamus to the primary sensory cortex. However, in people with MS (Figure 2B), while ascending through the spinal cord, fibres within the tractus gracilis are subject to inflammatory and degenerative processes that significantly reduce the number of fibres (and fidelity of message) arriving in the medulla. Subsequently, minimal plantar sensation is available for conscious and subconscious processes pertaining to balance and gait. It is hypothesized that when people with MS use the SurroGait Rx (Figure 2C), proprioceptive impulses that are encoded by the device and relayed wirelessly via Bluetooth™ to vibrating pads will transmit the information, encoded as vibrations, into the spinal cord by Ab-sensory nerve fibres at the upper thoracic and cervical levels of the spinal cord. This information will then travel through intact fibres of thetractus cuneteus to the nucleus cuneutus in the medulla where they will synapse on second-order neurons of the medial lemniscal pathway.
Figure 2: Schematic of pathway through the central nervous system for myelinated fibres carrying proprioceptive and vibratory information. The afferent sensory system is a 3-neuron pathway consisting of first-order neurons that carry impulses from the periphery up through the spinal cord to synapse in the medulla. The second-order neuron crosses midline and relays information to the thalamus. The third-order thalamocortical neuron carries information to the primary sensory cortex. Panels A, B, and C demonstrate intact CNS, MS, and MS + SurroGait™ scenarios, respectively.
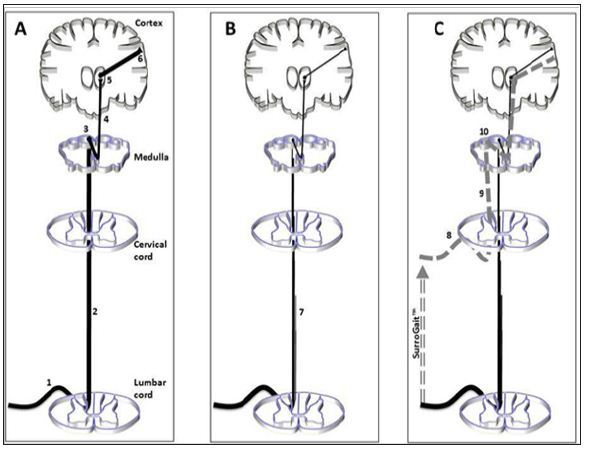
These fibres ascend to the ventral posterolateral nucleus of the thalamus where they synapse onto third-order neurons and then carry substituted sensory information to the primary sensory cortex. If successful, this device could have broad clinical applications, as improvements in walking and balance are associated with reduced fall risk and better quality of life [29]. Therefore, the objective of this study was to investigate the effects of training with the SurroGait Rx in individuals with MS with impaired plantar sensation, as well as to investigate the washout effects after ceasing the training protocol. It was hypothesized that individuals with MS would exhibit improvements in gait and balance, specificall lower MSIS-29 scores, faster walking speed and cadence, and reduced postural sway during standing after eight weeks of training with the SurroGait Rx. After a washout period, it was speculated that these improvements would depreciate.
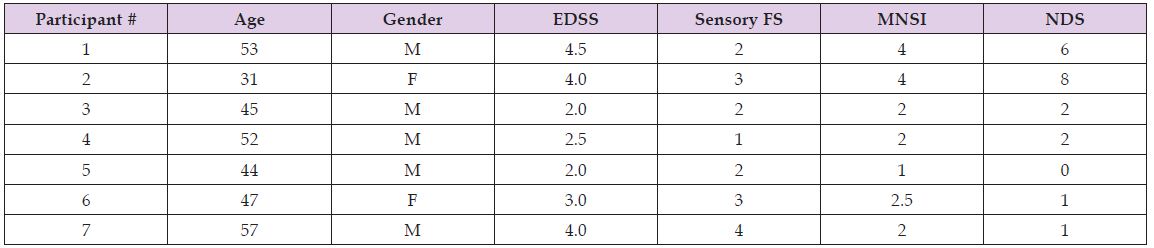
Seven individuals [age: 47.1 ± 8.6 years, height: 176.0 ± 12.9 cm, weight: 87.6 ± 30.8 kg (mean ± one standard deviation); (Table 1) with MS were recruited from a pool of participants at the Calgary Multiple Sclerosis Clinic by Dr. SJ. Pre-screening conducted by Dr. SJ and telephone interviews conducted by the research team confirmed participant eligibility in the study. The following inclusion criteria were met by all participants: 18 years or older; Relapsing-Remitting MS Impaired vibration/proprioception in at least one leg; At least mild wobble on Romberg testing Normal vibration and proprioception in both hands; All leg muscles have strength > 4/5 on manual muscle testing (MRC) Spasticity in both legs 2 on Modified Ashworth Scale No significant cerebellar ataxia (Cerebellar FS<2 on Expanded Disability Status Scale; EDSS); No large-fibre peripheral neuropathy (clinical or NCS); Ability to walk independently for at least 500 meters without aid or rest; No lower limb injuries in the last six months.
Table 1: Individual participant characteristics and scores on the clinical inventories to assess plantar sensitivity (EDSS: Expanded Disability Status Scale; MNSI: Michigan Neuropathy Screening Instrument; NDS: Neuropathy Disability Score).
As the inclusion criteria demonstrate, participants with predominantly sensory impairment were selected to increase homogeneity in the study population. All participants provided written informed consent in accordance with the University of Calgary’s Conjoint Health Research Ethics Board.
All participants visited the Human Performance Laboratory at the University of Calgary on four occasions. During visit 1 (familiarization), each participant was fitted for the SurroGait Rx device, completed the entire gait and balance protocol in order to mitigate learning effects, and completed two validated clinical Inventories to quantify their degree of neuropathy: the Michigan Neuropathy Screening Instrument (MNSI) and the Neuropathy Disability Score (NDS) [30]. These tests included manual inspection of the condition of the feet, testing sensitivity to pressure, temperature, vibration, and pain, checking for the presence of ankle reflexes, and a questionnaire of symptoms. All tests were conducted by the same individual, who had conducted similar tests in previous studies, but was not a clinician.
The tests differ in their ranges but are similar in that higher scores on each inventory correspond to greater severity of sensory dysfunction. Visits 2 (baseline), 3 (post-training), and 4 (washout) were identical and were scheduled one, nine, and 17 weeks after the familiarization session, respectively. At the completion of the baseline session, participants were given the SurroGait Rx device to take home for an eight-week training period. They were asked to use it actively while ambulating for at least one hour per day with no upper limit to its use. During the baseline session, participants were instructed in the use of the device. The participants were instructed to wear the vests in a snug, yet comfortable manner. The insoles were inserted into the shoes, and the insole’s transmitter cable ran up along the medial surface of the foot and the transmitter itself sat atop the shoe exterior, on the dorsum of the foot, secured by the laces. At the end of the 8 week post-training session, participants left the device with the researchers. During the following 8-week period, participants completed a washout phase, where they had no contact with the device until they returned for the final testing session (“washout”). In all test sessions, participants completed the following:
Impact of MS on the Patient: Participants completed the MSIS-29 psychological scale. The MSIS-29 is increasingly relied on as an outcome measure of the physical and psychological impacts of MS on the patient [31], and is supported by evidence of reliability, validity, and responsiveness, and is also known as a strong assessment for physical disability specifically in the MS population [32]. A minimal change in score of 8 points on the MSIS-29 has been recognized as clinically significant [33].
Biomechanical Assessment: Participants performed balance and walking tasks while wearing inertial measurement units (IMUs; Shimmer3, Shimmer, Dublin, Ireland). These devices feature accelerometers, gyroscopes, and magnetometers that track motion in three axes. While IMUs lack the rigor of traditional camera-based motion tracking systems, they offer a portability and flexibility that is more suitable for clinical and home-based (i.e. remote) research. Three IMUs were affixed to the participant using double sided tape. The first was attached to the sternum two centimetres below the suprasternal notch. This IMU recorded the sway of the torso and was the source of results for the balance tasks. The other two IMUs were mounted on each heel and provided information on the motion of each foot during the gait task.
Gait Task: Gait performance was assessed using the timed 25- foot walk (T25FW), a validated clinical inventory of pathological gait. The T25FW was selected because gait speed has been demonstrated to be a useful and reliable functional measure of walking ability, and the T25FW has high inter-rater and test-retest reliability [34]. Each participant walked a 25-foot over ground path four times for each of two device conditions – one with the device fully functional (“on”) and another identical except with the power turned off (“off”) – in a randomized order for a total of eight trials per assessment. The time to complete the course was measured electronically with timing lights (TC Timing System, Brower Timing Systems, Draper, Utah, USA). A clinically significant change in gait has been recognized as a 20% decrease in the T25FW [34].
Balance Task: To assess balance, subjects stood as still as possible in three different postural conditions for 60 seconds each:
a) Eyes open with feet shoulder width apart.
b) Eyes open with a narrow stance (feet touching on medial edges).
c) Eyes closed with feet shoulder width apart.
The original protocol called for eyes closed with narrow stance condition as well, but after pilot testing, and multiple comments about feeling unsafe in this pose, the condition was removed from the protocol to ensure participant safety. The width between the participant’s feet was standardized to 18 cm with masking tape in the first and third conditions. Participants were instructed to stand with their hands at their sides, to focus their gaze on a dot drawn on a whiteboard 4 m ahead, and to not move or speak for the duration of the trial. Each posture was repeated twice for each of the two device conditions (on/off) in a randomized order, for a total of 12 total balance trials per assessment session (3 postural conditions x 2 device conditions x 2 trials).
Balance performance was quantified through motion of the trunk during quiet standing tasks. The three-dimensional sway angle of the torso was calculated from a Kalman filter synthesis of the accelerometer and gyroscope data from the IMU mounted on the sternum. The total path length of the postural sway angle trajectory may be considered a metric of pathological balance control, [35] and it was speculated that a longer path length corresponded to worse balance (i.e., more trunk movement). The total length of the postural sway angles was derived from the middle 30 seconds of each 60-second balance trial. The accelerometers and gyroscopes in the IMUs mounted on the heel of the shoe were used to calculate the stride parameters. A stride is the period of gait from one heel strike to the next heel strike of that same limb. The variables of interest included the mean stride frequency (i.e. cadence) and variability of stride frequency, specifically its coefficient of variation.
All computations were performed with MATLAB software. Statistical analyses were made with SPSS software (IBM) using a significance level of α = 0.05. Statistical significance for the MSIS- 29 was assessed using a Friedman test with a post hoc analysis using the Wilcoxon signed-rank tests with a Bonferroni correction applied. Statistical significance for the T25FW, gait and balance variables were assessed with a 3 Day (baseline, post-training, washout) X 2 Condition (on, off) repeated measures ANOVA with a Bonferroni correction. All ANOVA tests satisfied the sphericity of variance condition (p > 0.05).
Neuropathy Inventories: Participants ranged from 1 - 4.0 on the B-portion of the MNSI scale, with an average rating of 2.5 ± 1.1 (Table 1). Participants ranged from 0-8 on the NDS scale, with an average rating of 2.9 ± 3.0. The individuals with the highest rating on the MNSI scale also had the highest score on the NDS scale.
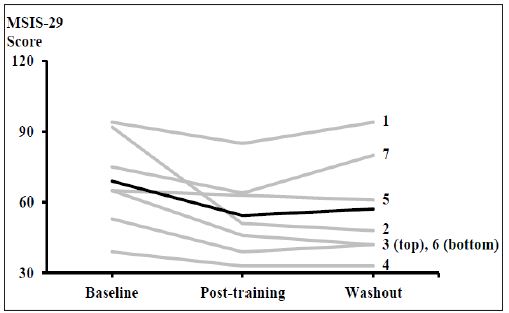
MSIS-29: MSIS-29 scores ranged from 33-94, with an average rating of 60 ± 20 over the three assessment periods (Figure 3). Five out of seven participants exhibited a clinically relevant improvement in their score from baseline to post-training, with an average decrease of 14.6 ± 12.9 points for all seven subjects. There was a statistically significant difference in the impact of MS on the patients across the three time points (χ2(2) = 7.154, p = 0.028). There were no significant pair wise comparisons; however there was a strong trend for a reduction in MSIS-29 score between baseline and post-training (Z = -2.366, p = 0.051).
Figure 3: Individual (grey) and mean (black) participant responses on the MSIS-29 questionnaire regarding the impact of MS on the patients. Individual participant numbers are indicated, and correspond to Table 1. A statistically significant difference was observed across the three times points (N=7).
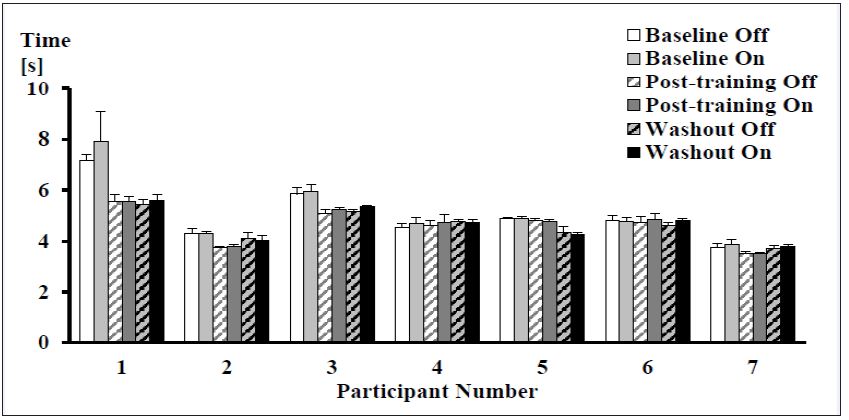
T25FW: For the T25FW, one participant reduced their average walking time above the clinically relevant threshold (≥ 20%) between the baseline and post-training sessions, however overall there were no significant differences in speed between device conditions or over time (Figure 4).
Figure 4: Mean (± SD) times to complete the timed 25-foot Walk test for each participant at every time point and device condition. No significant differences were observed (N = 7).
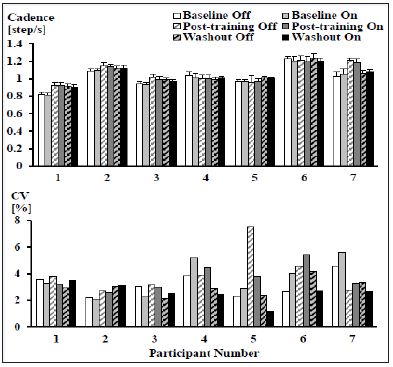
There were no significant differences in the mean stride frequencies or the coefficient of variation of stride frequency between device conditions or over time (Figure 5).
Figure 5: Mean (± SD) (top) and coefficient of variation (bottom) of stride frequencies for each participant across all time points and device conditions while walking. No significant differences were observed (N = 7).
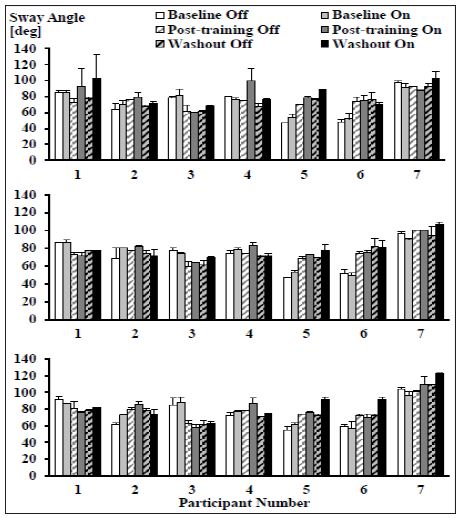
Total Path Length of Postural Sway: For all three postural conditions, there were no significant interaction effects or day effects. However, in all postures there were significant device effects (wide stance, eyes closed: F(1,7) = 10.327, p = 0.015; wide stance, eyes open: F(1,7) = 5.711, p = 0.048; narrow stance, eyes open: F(1,7) = 7.231, p = 0.031) with longer torso sway angle trajectories in the device on condition (Figure 6).
Figure 6: Mean (± SD) total lengths of postural sway angle trajectories across all time points and device conditions for the wide stance, eyes closed (top), wide stance, eyes open (middle), and narrow stance, eyes open (bottom) postures. In all postures there was a significant device effect with a longer torso sway angle trajectory in the on condition (N = 7).
The objective of this study was to investigate training effects of the SurroGait Rx on the physical and psychological impacts of MS on the patient, gait and balance. The key outcomes of this study indicate A decreased impact (i.e., improvement) of the disease post-training, No effects on gait speed, stride frequency or stride frequency variability, and Significantly longer postural sway trajectories when standing with the device on compared to off.
The most promising result from this study was the significant improvement regarding the reported effect of MS on the patient. Notably, five of seven participants showed clinically relevant improvements between baseline and post-training, and all seven participants showed decreased MSIS-29 scores after training. After the 8 week washout phase, the MSIS-29 scores remained mostly constant at this improved state, with five of seven participants deviating only ±4 points on the scale.
The remaining two participants showed an increase (worsening) of 9 points and 16 points. A limitation to the current study design with respect to the MSIS-29 questionnaire is that no control group was used. This leaves open the possibility that the self-reported improvement regarding the impact of MS on the patient was not a direct result of using the SurroGait Rx, but rather an increase in daily activity resulting from actively participating in a research study where frequent activity was encouraged. No statistical differences were found in any of the walking variables, either across testing days, or between device conditions (on/ off). This lack of improvement may be explained by the fact that the participant population had a relatively low burden of disease (average EDSS score was 3.1). As such, we may have encountered a ceiling effect, where there was minimal room for improvement in walking speed.
The single subject who did demonstrate a clinically-significant improvement on the EDSS was the only subject with a baseline T25FW time > 6 seconds, and also had high scores on the MNSI and NDS tests. A T25FW time > 6 seconds has been previously identified as a benchmark for clinically-meaningful disability [36]. Ultimately, these data argue that further research should target individuals with MS with a larger burden of disease (and longer T25FW), in order to better investigate the effects of the SurroGait Rx. Fampridine, a medication approved for improving walking speed in PwMS, is approved for EDSS 4-7, which also suggests a lack of efficacy in subjects who lack a significant deficit with respect to walking speed. Another consideration for this device would be to use a gait assessment of longer duration, which may be more sensitive to small changes in gait speed. Possible tests include the 6-Minute Walk Test or the 2-Minute Walk Test, both of which have proven feasible and reliable in an MS population [37,38].
The total path lengths of the postural sway angle trajectories were significantly greater when the SurroGait Rx was turned on, versus off for all quiet standing conditions. This finding contradicts the initial hypothesis of a reduced sway angle trajectory when using the device. Panzer et al. utilized posturograpy to do a detailed biomechanical assessment of the changes in balance seen with aging [39]. They reported that an increased amount of sway (longer path length) did not correlate with any evidence of postural instability. What they observed was the adoption of a different postural control strategy - larger corrective movements versus continuous small adjustments. They concluded that increased amplitude of postural movements suggested a decrease in the subject’s vigilance to the maintenance of upright posture. This is also discussed in a more recent paper [40], where the notion that sway correlates with balance (and thus poor balance is observed as a longer path length) cannot be applied when different balance strategies are employed. In that study, it was found that increased path length corresponded to a larger hip strategy versus a continuous ankle strategy for maintaining balance.
As a result, SurroGait-Rx could qualify as a ‘different balance strategy’ and the increased path length may be a sign that a novel balance strategy is being employed in people wearing the device. For example, when the device was on, participants would consciously tolerate a greater sway excursion, knowing that the device would signal to them when they reached the limits of their correctable balance by way of higher intensity vibrations on the lateral edges of the vest (corresponding to high pressures on the outer edges of the insoles). In essence, they had a reliable ‘secondary signal’ to tell them when they were reaching the limit of a safe distance from their centre of gravity.
The finding of a spontaneous effect on balance with the device on, coupled with the lack of significant longitudinal training effects suggests that the influence of the SurroGait Rx on balance in this short-term study, is instantaneous rather than achieved after training. This may argue that the effect of the device is not mediated by afferent sensory information traveling to higherorder CNS sensory systems via the dorsal columns, but rather is mediated by information flowing into the cerebellum via spinocerebellar pathways. This is supported by research showing that the cerebellum is primarily involved in sensory data acquisition (particularly proprioceptive and tactile information), and is capable of inducing instantaneous effects on motor pathways [41]. Thus, a future study including MS subjects with both mild and significant cerebellar involvement will be particularly informative.
One possible adverse outcome of the trial was that dependency on the SurroGait Rx that may have developed during the 8-week training period either due to Neuroplasticity or familiarity with the device, would subsequently lead to worsened function after the 8-week washout. The data support the notion that dependency did not occur. This further argues for the safety of the SurroGait Rx device. A potential confounding factor to this study was differences in training volume between individuals, as well as differences in types of activities conducted while wearing the SurroGait Rx. It is currently unknown how these training differences affect the motor control adaptations, and thus the results of the study. Another limitation was the small sample size used, making it difficult to make inferences and interpretations regarding the data. However, this small scale study did elicit enough promising outcomes to warrant further investigation of this device.
Individuals with MS exhibited significant reductions in the physical and psychological impacts of the disease after training with the SurroGait Rx for eight weeks. Subjects also demonstrated instantaneous changes in balance while using the device, but no changes in gait were observed. It is recommended that future research be conducted on people with greater disability, who have greater potential for improvement.
The authors would like to thank all the participants for completing the study. This work was supported by funding from the University of Calgary Department of Clinical Neurosciences and Orpyx Medical Technologies Inc.
This study was designed, conducted, and interpreted with no influence from the funding sources, including Orpyx Medical Technologies Inc.


