Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Farahnaz K Behbahani* and NajmehDashtaki
Received: January 03, 2018; Published: January 09, 2018
*Corresponding author:K Behbahani, Department of Chemistry, Karaj Branch, Islamic Azad University, P.O. Box 314/85313 Karaj, Iran
DOI: 10.26717/BJSTR.2018.02.000655
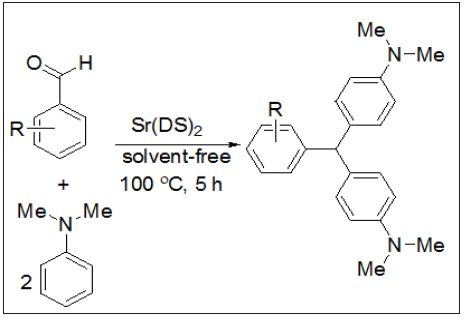
A fast, efficient and versatile route for the synthesis of 4,4’-diaminotriarylmethanes using Sr(DS)2 as a Lewis acid-surfactant-combined catalyst, LASCs in the presence of N,N-dimethyl aniline and aryl aldehydes is reported. LASCs not only act as a Lewis acid to activate substrate molecules, but also serve as a surfactant to form stable colloidal dispersion systems with organic substrates. Also, the others advantages of LASCs are easy separation and recovery, high activity, selectivity, as a Lewis acid to activate the substrate molecules and as a surfactant to help to solubilize the organic compounds in water. In this project a variety of aryl aldehydes were subjected to synthesize of 4,4’-diaminotriarylmethanes in the presence of strontiumdodeceyl sulfate, N,N-dimethyl aniline under solvent free condition and at 100 °C%.
Keywords: Sr(DS)2; N,N-dimethyl aniline; Aldehyde; Catalyst; Surfactant y
Dyes may be classified according to chemical structure or by their usage or application methods. According to the chemical structure classification, triarylmethane dyes are among the most important dyes. Triarylmethane dyes are monomethine synthetic dyes with three terminal aryl groups, of which at least one, but preferably two or three, are substituted by a donor group para to the methane carbon atom. Due to the importance of N,N-dimethyl aniline (DTM) compounds, several reviews have disclosed on diand triphenylmethane derivatives [1-5]. These compounds have a broad range of applications in color-forming, manufacturing of novel types of various colorless copying papers, pressure-sensitive heatsensitive materials, high-speed photo duplicating copying papers, light-sensitive papers, ultrasonic recording papers, electrothermic heat-sensitive recording papers, inks, crayons, typewritten ribbons, and photo imaging systems [6]. Accordingly, different methods for the preparation of the aforementioned compounds have been described such as those from arene nucleophiles and triethylorthoformate, or benzhydrol in the presence of acid catalysts [7-10] from condensation of amines and anilines using acids [11] or zeolites [12] and also by reaction of aniline derivatives with metal catalysts such as Pd(OAc)2 [13] or clay-mediated oxidative coupling of di substituted anilines using microwave radiation, in which DTM derivatives were obtained in low to moderate yields [14].
One of the most useful methods for the synthesis of DTM is the reaction of arylaldehydes with N,N-dimethylaniline in the presence of an acid such as sulfuric acid, HCl, p-TSA, zeolites or montmorillonite K-10 [14-19]. Although different methods for the preparation of the aforementioned compounds have been described, most of them however, suffer from drawbacks such as the use of corrosive acids or toxic or hazardous chemicals, excess of solvents and harsh reaction conditions, which will result in generation of waste streams, complicated workup procedures, byproducts and isomeric mixtures and consequently, low yields. Therefore, there is still a demand to search for a better catalyst with regards to toxicity, selectivity, availability and operational simplicity for the synthesis of tri arylmethane compounds. LASCs (Lewis acid-surfactantcombined catalyst) firstly developed by Kobayashi [20,21] as one of the most widespread investigated catalytic system, has attracted increasing attentions during the past decades. Not only LASCs act as a Lewis acid to activate substrate molecules, but also serve as a surfactant to form stable colloidal dispersion systems with organic substrates.
Most reports on LASCs enhanced on their uses in organic reactions such as aldol, Diels–Alder, allylation of Aldehyde, propargylamines reactions, asymmetric hydroxylation, hydroxymethylation, ring– opening reactions and so on [22]. Also, due to easy separation and recovery, high activity, selectivity, as a Lewis acid to activate the substrate molecules and as a surfactant to help to solubilize the organic compounds in water, there is still demand for developing LASCs and its usage in organic transformations. this communication, we wish to report a green synthesis method for the preparation of DTMs using arylamines and aldehyde derivatives in the presence of strontium dodecyl sulfate as LASCs (Figure 1). Photocatalyst has been widely applied in the treatment of dye pollutants in wastewater [1-3]. Until now, various kinds of compound materials, such as metal oxide and nanocomposites have been widely used as a photocatalyst [4-8]. Many researchers have been reported the properties of semiconductors depend on their crystal structure, size and morphology [9,10]. Therefore, achieving high photocatalytic activity is still a challenge. Until now, different methods for synthesis of Zn2SnO4 and SnO2/Zn2SnO4 have been reported in literature? [11-13].
Figure 1: Synthesis of 4, 4’-diaminotriarylmethane using Sr(DS)2.
Melting points were measured by using the capillary tube method with an electro thermal 9200 apparatus. IR spectra were recorded on Perkin Elmer FT-IR spectrometer did scanning between 4000–400 cm–1. 1H NMR spectra were obtained on Bruker DRX- 300MHz NMR instrument in CDC13. Analytical TLC of all reactions was performed on Merck precoated plates (silica gel 60F-254 on aluminium). All compounds are known and spectra and physical data were compared with those of authentic samples [23,24].
To a stirring solution of sodium dodecyl sulfate (40 mmol, 11.51g) in distilled water (300 mL) was added a solution of Sr(DS)2(10 mmol, 2.11g) in water (100mL) at room temperature. A white precipitate appeared immediately and the mixture was stirred for another 30 min. The white solid was separated by filtration and washed with water (2 × 150mL). The isolated solid product was dried under reduced pressure and Sr(DS)2as a white powder was obtained in 87% yield (10g). Mp. 168°C(dec).
A vial equipped with a stir bar was charged with aryl aldehyde (0.5 mmol, 1eq), N,N-dimethyl aniline (1.5mmol, 3eq) and Sr(DS)2 (20 mol %) and the vial was capped. The resulting mixture was heated in an oil bath at about 100 °C for 5-6 h, and the course of the reaction was monitored by TLC on silica gel with ethyl acetate: n-hexane (1:4) as eluent. Finally, the reaction mixture was cooled, dichloromethane (15ml) was added and the catalyst was removed by filetaration. After evaporation of the solvent, the crude product was resulted and crystallized from ethanol.
After the completion of the reaction, dichloromethane (15ml) was added to the reaction mixture, and then, the catalyst was separated by filtration. The precipitate was washed with dichloromethane and then air dried for the catalyst reusability. The catalyst was recycled for the synthesis of dimethyl-4-[[4- (dimethylamino) phenyl](4-Chlorophenyl)methyl] aniline three times (95, 89, 85)% entry 3, (Table 3).
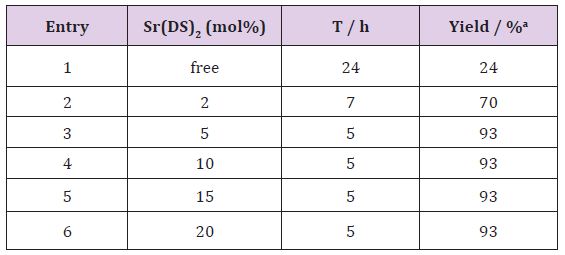
Our initial attempts to test the feasibility of the reaction employed 4-chlorobenzaldehyde and N,N-dimethyl aniline under various reaction conditions. Firstly, optimization of different solvents was studied in the benchmark reaction. The results were revealed, that the catalyst more efficiently worked under solventfree conditions at 100 °C (Table 1). The authors believe strontium dodecyl sulfate as LASCs homogenizes reaction mixture in the absence of solvent. Yields of the isolated products from the reaction of 4-chlorobenzaldehyde (1.5 mmol, 0.2 g), N,N-dimethylaniline (4.5mmol, 0.54 g) Sr(DS)2 (5 mol%). Also the effect of catalytic amount of Sr(DS)2on the model reaction was investigated. It was found that amount of the catalyst played a major role in establishment of the desired product yield. The best optimized reaction condition was found at 5 mol% of Sr(DS)2under solventfree conditions at 100 °C (Table 2). Yields of the isolated products from the reaction of 4-chlorobenzaldehyde (1.5 mmol, 0.2 g), N,Ndimethylaniline (4.5 mmol, 0.54 g) Sr(DS)2 (5 mol%).
Table 1: Optimizing of kind of solvent and temperature in the synthesis of dimethyl-4-[[4-(dimethylamino) phenyl] (4-Chlorophenyl)methyl] aniline.
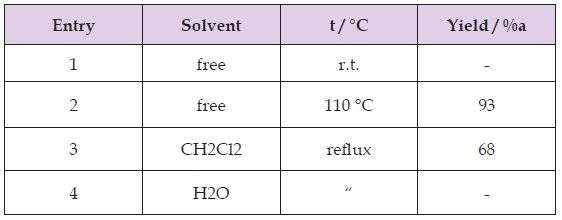
Table 2: Optimizing of the catalyst amount in the synthesis of dimethyl-4-[[4-(dimethylamino)phenyl](4-Chlorophenyl) methyl] aniline
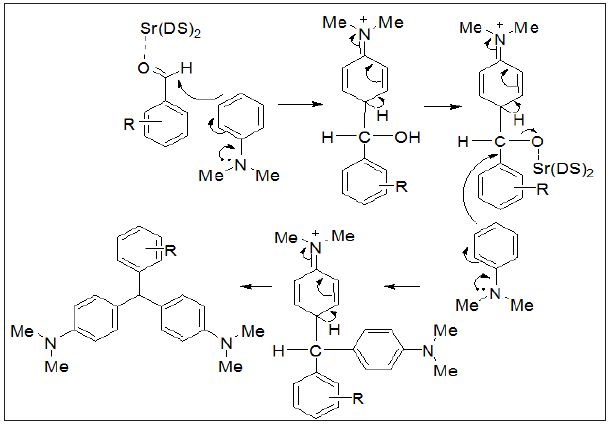
Therefore, a wide variety of substituents were subjected on the arene, in ortho-, meta- and para-positions. The aryl aldehydes with electron-withdrawing groups such as halo-substituents at the paraand nitro-substituents at the ortho-, meta-, and para-positions gave excellent yields. However with electron-donating groups such as methyl and N,N-dimethyl amino substituents, lower yields of the desired products were obtained due to decrease the electrophilicity of carbonyl group in the corresponding electrophilic aromatic substitution reactions (Table 3).Proposed mechanism for the synthesis of 4,4’-diaminotriarylmethane has also been depicted in (Figure 2).
Table 3: Synthesis of 4,4’-diaminotriarylmethane using aromatic aldehydes, N,N-dimethyl anillin and Sr(DS)2.
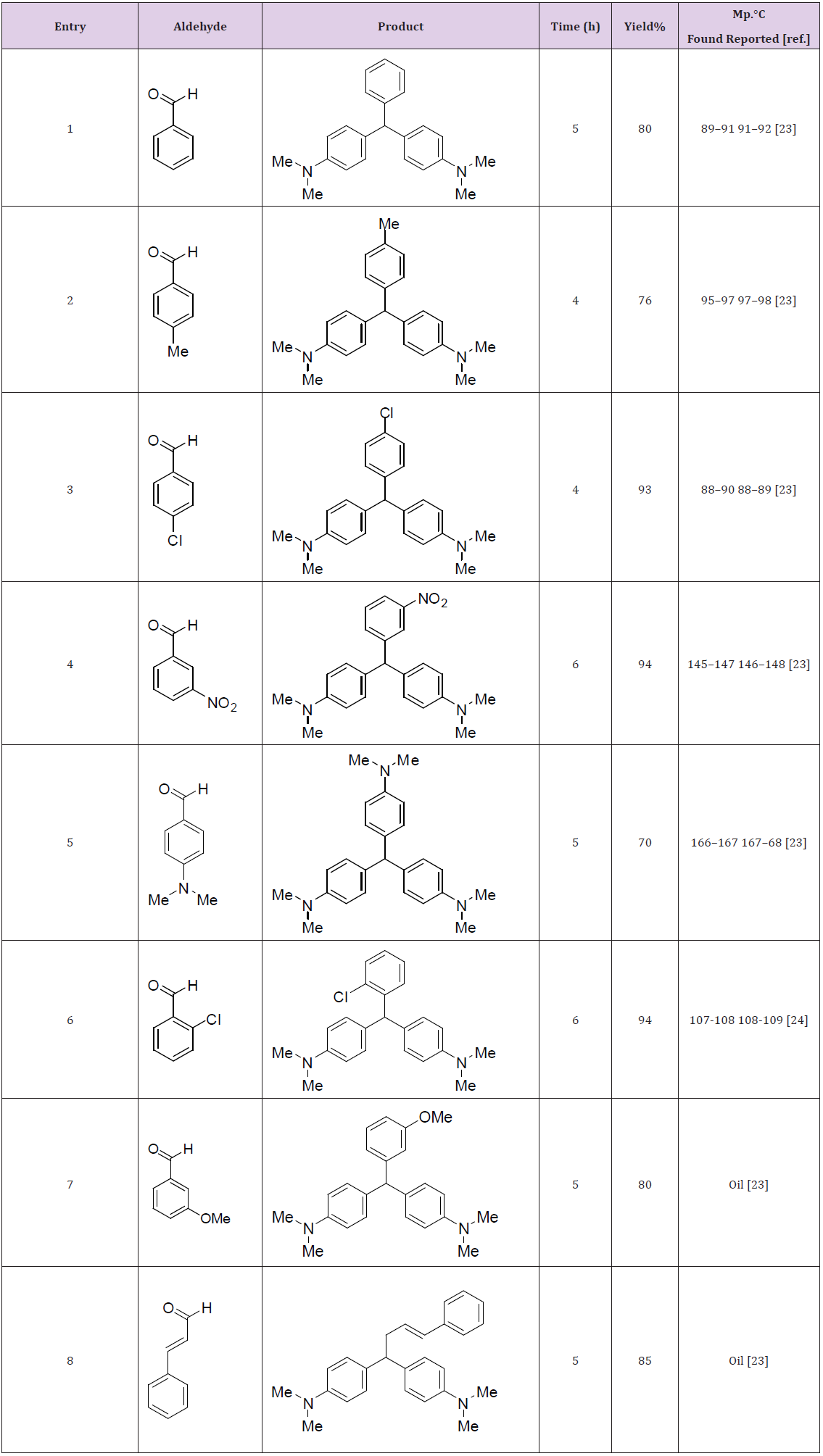
Figure 2: Proposed mechanism for the synthesis of 4,4’-diaminotriarylmethane has also been depicted.
In summary, the authors have developed a simple, efficient and green methodology for the synthesis of diaminotriarylmethaneleuco base materials using a catalytic amount of strontium dodecyl sulfate under solvent-free conditions. The simple experimental procedure, solvent-free reaction conditions, and good yields, easy separation and recovery of the catalyst are the advantages of the present method.


