Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Aman Kugasia*1, Ailda Nika1, Winston Sequeira1, Joel Block1, Meenakshi Jolly1, Chandrahasa Annem2 and Palashkumar Jaiswal3
Received: December 07, 2017; Published: December 15, 2017
Corresponding author: Aman Kugasia, Rush University Medical Center, University Rheumatologist 1611 West Harrison Street, Suit 510, Chicago- IL- 60612
DOI: 10.26717/BJSTR.2017.01.000597
Studies have demonstrated the role of TH-17 pathway in the pathogenesis of Systemic Lupus Erythematosus (SLE). Ustekinumab, a monoclonal antibody, binds and inhibits p40 subunit of IL-12 and 23, leading to IL-17 blockage, causing inhibition of TH-17 signaling pathway. Ustekinumab has rarely been used in the treatment of SLE. Herein, we discuss a patient with long standing history of Psoriasis and Psoriatic Arthritis, which was successfully being managed with anti TNF therapy. He was later diagnosed to have concomitant SLE. His persistently active and aggressive psoriatic lesions, arthritis and thrombocytopenia posed an interesting therapeutic dilemma. Fortunately his psoriatic lesions, arthritis and thrombocytopenia responded well to ustekinumab. Methotrexate was added to his regimen after improvement and he remained in remission on this therapy. Further clinical studies are warranted to investigate the role of TH-17 blocking agents in SLE.
Keywords: Ustekinumab; Systemic Lupus Erythematosus (SLE); Psoriasis; Thrombocytopenia; IL 17; IL 23
Abbreviations: SLE: Systemic luous erythematosus; CCLE: Chronic Cutaneous Lupus Erythematosus; DLE: Discoid Lupus Erythematosus; PsA: Psoriatic Arthritis; Ps: Psoriasis; TNF: Tumor Necrosis Factor; IL: Interleukin; AIHA: Auto Immune Hemolytic Anemia; PUV-A: Photo Ultraviolet A; Anti Ds-DNA: Anti-Double Stranded Deoxyribonucleic Acid; LAC: Lupus Anticoagulant; Anti RNP: Anti- Ribo Nuclear Protein; Anti Sm: Anti- Smith; MRI: Magnetic Resonance Imaging; Mg: Milligrams
Interleukins (IL) are involved in the pathogenesis of several autoimmune disorders such as rheumatoid arthritis, crohn’s disease and psoriasis (Ps), and specific IL blockade has been utilized successfully to treat patients with these diseases [1-2]. The role of the p40 subunits of IL-12 and IL-23 is increasingly being explored in the pathogenesis of systemic lupus erythematous (SLE) [3-7]. Ustekinumab is a human monoclonal antibody that binds and inhibits the p40 subunit of interleukins 12 and 23, which are involved in the TH-17 signaling pathway. It is approved for the treatment of moderate to severe plaque Psoriasis [8]. While there have been isolated case reports of its successful use for the treatment of refractory cutaneous lupus (non SLE), suggesting that targeting the TH-17 pathway may be useful in this condition, its use in SLE remains poorly described [9-10]. Here, we report a case of successful use of Ustekinumab in a patient with active SLE and psoriasis (Ps).
Our patient is a 48-year-old man with history of psoriasis (Diagnosed in 2001), autoimmune hemolytic anemia (AIHA) treated with rituximab (diagnosed in 2006 and rituximab given in 2006), who presented to the rheumatology clinic (2008) for management of psoriatic arthritis (PsA). His psoriasis has only partially responded to topical isotretinoin, PUV-A and etanercept. Physical examination showed erythematous, scaly plaques involving the lower extremities, lower back, and the extensor surfaces of both elbows. His left knee was warm, tender, and non-erythematous with a moderate sized effusion. Synovial fluid analysis showed 18,000 wbc/μL (87% neutrophils) without crystals. The gram stain was negative. Laboratory evaluation showed thrombocytopenia (52,000 cells/μL), positive anti-nuclear antibody (ANA) (1:640), anti-double stranded DNA antibody (Anti Ds-DNA) (42 IU), anti SS-B antibodies at 1.18 (≥ 1.10 is considered Positive) and lupus anticoagulant (LAC). Anti ribonuclear protein (RNP), anti- Smith(Sm), anti-histones, anti-SS-A, anti β-2 glycoprotein and anti cardiolipin antibodies were negative. His White cell count was at 9200 cells/μL & hemoglobin was stable at 14g/dL.
Comprehensive metabolic panel, complements and urinalysis were within normal range. Knee radiograph revealed mild narrowing of the lateral patello-femoral compartment. MRI of right knee showed mild enthesopathy of anterior patella). infliximab 500mg every 8 weeks. A diagnosis of psoriasis, psoriatic arthritis (oligo-articular asymmetric arthritis pattern with inflammatory synovial fluid) was made. Patient was started on etanercept 50mg every week subcutaneously (5/2010). His skin disease and arthritis did not improve after 3months on this medication so he was switched to infliximab 500mg every two weeks subcutaneously. In retrospect he was diagnosed with SLE clinically based on positive ANA, anti-Ds-DNA, positive lupus anticoagulant, AIHA that responded to rituximab and thrombocytopenia. Concern for the drug-induced lupus was low because AIHA preceded any biological agent administration and thrombocytopenia preceded infliximab administration. Infliximab was discontinued on the side of caution. He was substituted to mycophenolate mofetil. Patient’s skin lesions, synovitis of knees and platelets did not improve after 6 months therapy with mycophenolate 500mg twice a week and prednisone 10mg daily. He was switched to cyclosporine 300mg daily along with prednisone.
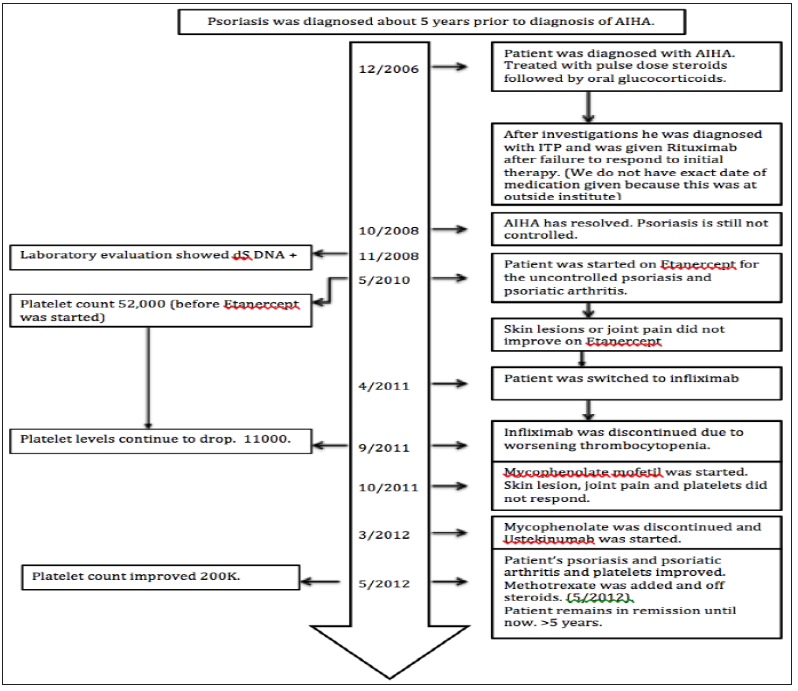
This regimen improved his psoriatic skin lesion (Figure 1) but he had persistent arthritis, recurrent inflammatory knee effusions and low platelets. We initiated ustekinumab 90mg subcutaneously at 0, 4 week and then every 12 weeks and cyclosporine was stopped. After three doses of ustekinumab, there was complete resolution of skin lesions and normalization of platelet count (200,000 cells /μL). Methotrexate 10 mg every week was added given persistent arthritis. After four months of therapy with ustekinumab and methotrexate, prednisone was tapered off. Patient had been on the current regimen for the last five years without recurrence of thrombocytopenia, AIHA, psoriatic skin lesion or arthritis. No medication related adverse effects have been noted. Timeline of events have been depicted in chart form in Figure 2.
Figure 1: Panel A showing psoriatic plaques on elbow and leg prior to treatment with Ustekinumab. Panel B showing complete resolution of skin lesions after treatment with Ustekinumab.
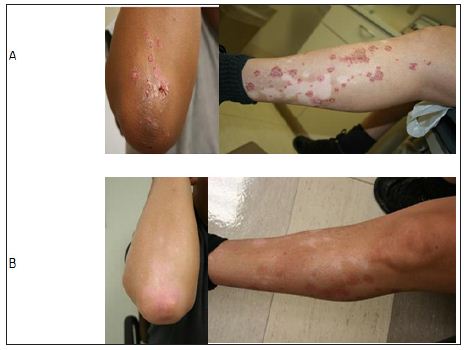
Figure 2: Timeline for the events.
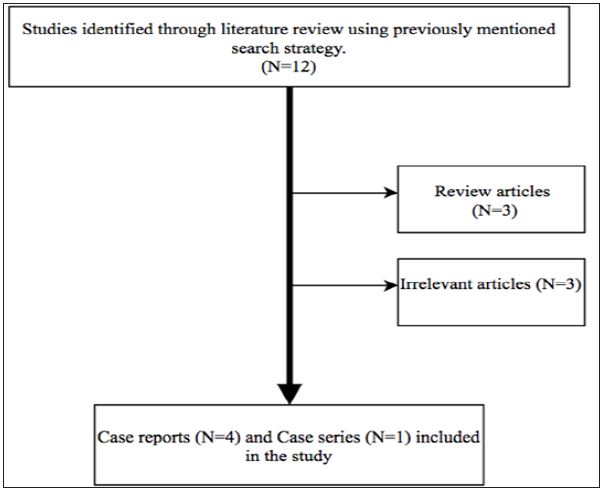
This systematic review was conducted according to the PRISMA guidelines. A computer-assisted literature search of PubMed and Ovid search engine was conducted. In order to increase the sensitivity of our search, we used free text words and MeSH terms with and without Boolean operators (“AND” and “OR”). Search terms keywords to identify different types of systemic and cutaneous lupus included “SLE”, “systemic lupus erythematosus”, “discoid lupus”, “acute cutaneous lupus erythematosus”, “subacute cutaneous lupus erythematosus”, “discoid lupus” and “chronic cutaneous lupus erythematosus”. We came across 85347 cases of different types of lupus when we used “OR” Boolean operator. We associated these 85347 studies with “ustekinumab”, “IL-12 inhibitor” and “IL-23 inhibitor” and the output was 12 studies. Out of these 12 only 5 studies fulfilled the inclusion criteria and were included in the study (Figure 2).
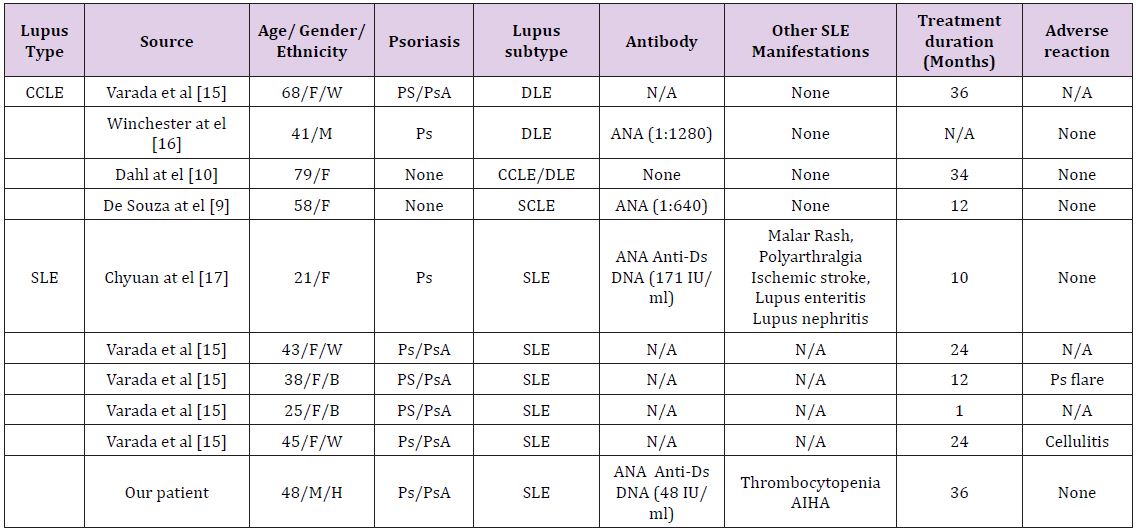
No language restrictions were enforced. The inclusion criteria for our systemic review were rather liberal. We included all the human studies that enrolled patients with different kinds of lupus treated with Ustekinumab. Other major inclusion criteria were minimum age of more than 18 years, SLE, CCLE and DLE patients who have received Ustekinumab. Exclusion criteria included review articles, basic science research, animal studies, irrelevant articles in which either the patient did not have any kind of lupus or in whom ustekinumab was not used for treatment. Following data were extracted and compared for all the studies: lupus type and subtype, age, gender, ethnicity, lupus clinical manifestations, ANA antibody levels, treatment duration and adverse reaction (Figure 3 and Table 1).
Figure 3: Search strategy for literature search.
Psoriasis (Ps) is a relatively common disease affecting 1% to 3% of the US population, whereas SLE is significantly less common with reported prevalence rates in the United States ranging from 20 to 150 cases per 100,000 for SLE [11-12]. Cases of coexistent Ps and SLE are uncommon [13]. In one of the largest studies to date, it has been reported that cutaneous lupus manifestations (CLE) with or without SLE can occur in 0.69% of patients with Psoriasis and 1.1% of patients with CLE with or without SLE had concomitant psoriasis. Of these patients that had both, about 50% had SLE of which 64% also had cutaneous Lupus manifestations as part of SLE [14-15]. Three case reports of successful use of Ustekinumab in cutaneous Lupus Erythematosus without systemic involvement suggest that Th-17/ IL-23 may be involved in the pathogenesis of CLE [9,10,16]. Unlike our patient who had ongoing active SLE manifestations (thrombocytopenia), Chyuan et al reported a case of well controlled SLE and biopsy proven lupus nephritis, that developed resistant psoriasis, wherein Ustekinumab was successfully used and tolerated well, indicating its role in SLE [17].
In a recent retrospective chart review, of 96 patients with concomitant psoriasis and CLE with or without SLE (90% of them had SLE), 5 patients were treated with Ustekinumab [15]. Four were maintained successfully on the drug, and one experienced loss of efficacy. Two of these patients had SLE with chronic plaque psoriasis, 2 had SLE, chronic plaque psoriasis and psoriatic arthritis, and 1 had discoid lupus erythematosus (DLE) with chronic plaque psoriasis. Improvement of all, cutaneous psoriasis, cutaneous SLE symptoms (malar and discoid rashes) lupus arthritis, oral ulceration, and hematologic abnormalities (thrombocytopenia or anemia) were reported among these patients. Although this study was not able to clearly discriminate between the improvements noted in the arthritis of psoriatic or lupus it showed that the patients on Ustekinumab had better outcomes. Although psoriasis and SLE have relatively different pathophysiologic mechanisms, they both share up regulation of the Th17 immune pathway with elevated levels of interleukin (IL)-17, IL-23, and IL-12 [18]. IL-17 & IL-23 have been associated with the pathogenesis rheumatoid arthritis (RA), inflammatory bowel disease, ankylosing spondylitis and SLE [1-2]. Although not observed in all studies, serum IL- 17 levels have been found to be elevated in SLE compared to controls [19].
Correlations between serum IL-17 levels and SLE disease activity and anti-double stranded DNA (anti-dsDNA) antibody levels have previously been reported in some studies [5-6]. IL-12, elevated in SLE, stimulates the production of gamma interferon by TH-17 lymphocytes leading to glomerular nephritis and vasculitis. Serum p40 titers (IL-12 subunit) have been reported to be significantly higher in SLE patients compared to those of healthy subjects and patients with RA [20-21]. In SLE patients, serum p40 levels were positively correlated with the SLE disease activity index (SLEDAI) [21]. Ustekinumab that binds and inhibits p40 subunit of interleukins 12 and 23, which are involved in the TH-17 signaling, should be a potentially useful drug in the treatment of psoriatic arthritis with CLE or SLE. This case highlights the therapeutic challenges in managing a patient with concurrent psoriasis and SLE. Ustekinumab has been used frequently in treatment of psoriasis and psoriatic arthritis. However, its use is largely limited to a few case reports in patients with either CLE or in patients with psoriasis with coexistent CLE. Herein, we report a patient with active psoriasis, active psoriatic arthritis and active SLE, who responded well to ustekinumab, there by suggesting the potential role of TH-17/ IL23 pathway as therapeutic target in treatment of cutaneous (non SLE) lupus and SLE. Further clinical studies are warranted in order to investigate the role of Th-17 blocking agents in SLE.
Table 1: Overview of all the existing case reports of patients who have been treated with Ustekinumab for cutaneous lupus or SLE & Psoriasis or cutaneous lupus or SLE alone.
Abbreviations: M: male, F, Female, W: White, B: Black, H: Hispanic, N/A: Not available


