Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Kazemipoor Maryam *
Received: November 17, 2017; Published: December 05, 2017
Corresponding author: Kazemipoor Maryam, Assistant Professor, Department of Endodontics, School of dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
DOI: 10.26717/BJSTR.2017.01.000566
Nowadays, the application of Nanobiomaterials in dentistry is increasing. The combination of biomaterials with nanotechnology leads to the term of “Nano biomaterial”. Nanotechnology aims to control over material properties at nanoscale and over the precision and sensitivity of various tools and devices in different technologies. In the endodontic science, Nanobiomaterials are utilized in different aspects such as: instruments and materials modifications, improvement of root canal disinfection and obturation, root repair materials, local anesthesia and repair and regeneration of pulpal tissue. The aim of the present study was to reviews the research findings and future possible applications of Nanobiomaterials and nanotechnology in endodontics.
Keywords: Endodontic; Nanomaterial; Nanotechnology
Dental pulp is a unique low compliance tissue with specific characters different from similar loose connective tissues. Its encasement within the relatively rigid and unyielding dentinal walls, its particular rich neurovascular supply, a powerful immune response and scarcity of collateral circulation may lead to rapid degeneration and necrosis [1]. Dental caries, traumatic injuries and iatrogenic procedures could affect the health of dental pulp and trigger immune response within the pulpo-dentin complex [2,3]. Bacterial byproducts and products from the dissolution of the organic and inorganic constituents of dentin, mechanical and thermal injuries during cavity preparation, toxicity of restorative materials and more importantly micro leakage at the interface of dentinal walls and restorative materials could alter the existent balance within the pulp and cause irreversible pulpitis and pulp necrosis [4,5].
Clinical endodontics is mainly directed towards curing or preventing apical periodontitis. The microbial infection of the pulp via their toxins and noxious metabolic byproducts, in addition to the presence of disintegrated pulpal tissue are the primary causes of apical periodontitis [6]. Nonsurgical root canal treatment has a high degree of predictability with favorable outcome rates of up to 95% for the treatment of teeth with irreversible pulpitis [7] and up to 85% for necrotic teeth [8]. Endodontic treatment is based on the main two integrated phases: cleansing and shaping [9]. Cleansing and shaping procedures are directed towards the mechanically debridement, disinfecting the root canal system with irrigants and medicaments, and finally optimized canal geometrics for adequate obturation and seal [10,11]. The mechanical debridement is aimed to prepare all the root canal surfaces in a fully incorporated form into the original canal shape. Moreover, preparation errors such as perforations, zips, transport and etc. should be absent and as much as radicular dentin should be left to avoid vertical root fracture [12,13].
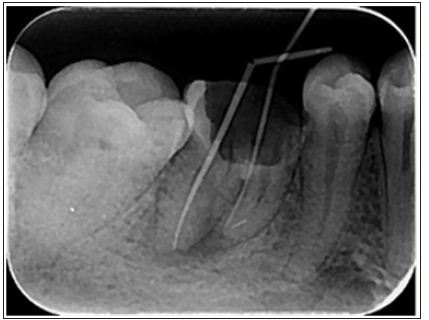
Endodontic instruments, both hand- held and engine-driven, are available for root canal preparation. Since the early 1990s, with the advent of nickel-titanium, various instrument designs and modalities have been produced in this regard [14]. Surface quality is an important factor in the function and durability of NiTi instruments and superficial defects such as metal flash, roll over and cracks may lead to the instrument fracture [15] (Figure1). Electro polishing the surface and coating it with titanium nitride have been recommended for promotion of the surface quality [16,17]. Currently, nanomaterials, with a smaller size, are being suggested for surface modification and reduction the incidence of failure in the rotary nickel-titanium files [18].
Figure 1: A separated nickel-titanium instrument in the mesiobuccal canal of mandibular first molar.
Nanoscaffolds for pulp regeneration, bioceramics for retrofilling, and repair materials are other applications of nanotechnology in the endodontic treatment (Figure 2). Nanorobots and nanoterminators are also new technologies for local anesthesia with fewer side effects and complications [24]. Nanotechnology has revolutionized all aspects of science and endodontic is no exception. Nano sized particles with significantly superior properties compared to the similar materials at larges scales of measurement have improved the quality of treatment. Understanding of dental tissue at the nanoscale, enabling the precise design of materials and instruments with ultrafine architecture and improving the present techniques in clinical dentistry have significantly promoted the quality of treatment.
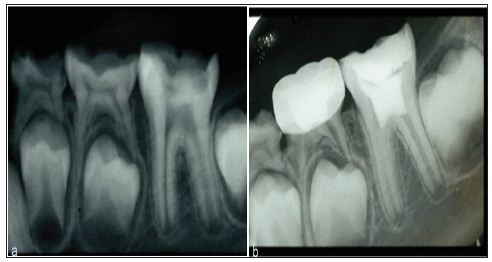
Figure 2: Deep pulpotomy for apexogenesis. a. Extensive carious exposure in an immature mandibular first molar with a history of spontaneous pain. b. After complete caries removal and hemostasis, the radicular pulp is overlaid with mineral trioxide aggregate (MTA).
Two concerns remain in this regard:
a) Nanotechnology should make its way from laboratories to clinical practice.
b) The significant potential for misuse and abuse of this technology on a scale and scope should not be overlooked.


