Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Rakesh KE and Rosy Antony*
Received: November 15, 2017; Published: December 01, 2017
Corresponding author: Rosy Antony, PG and Research Department of Chemistry, Nirmalagiri College, Kuthuparamba-670701, India
DOI: 10.26717/BJSTR.2017.01.000558
Modified chicken feather has been used as biosorbent for removing chromium ions from aqueous solution. The adsorption capacity tests were performed on an equilibrium batch basis. The parameters such as contact time, biosorbent dose, pH etc were optimized and were found to be 2 hrs, 100 mg & pH 6.0. Equilibrium isotherms were analyzed for the adsorption process and Freundlich adsorption isotherm model was found to fit the data well. The kinetics of adsorption followed pseudo-second order model.
Keywords: Modified Chicken Feather; Bio sorption; Chromium ions; Freundlich isotherm; Pseudo second order
Heavy metals such as Hg, Pb, As, Cr, etc are harmful to soil, water bodies and aquatic life. They are leached out mainly from industrial wastes, mines and research labs [1]. They produce acute toxicity in plants, animals and micro organisms. Due to bio accumulation and bio magnification through food chain they cause chronic effects even at lower concentrations. Heavy metals are generally removed from environment by precipitation, reverse osmosis, coagulation, flocculation etc [2-7]. These processes have many drawbacks, which include selective or partial removal of metal ions and high operational cost. Bio sorption can be used for effective removal of the heavy metals from environment. The major advantages of biosorption are low cost, high efficiency, regeneration, metal recovery etc. Bio sorption is largely influenced by pH, the concentration of biomass and the interaction time.
A very big problem of the agriculture industry is managing the enormous amount of waste generated by poultry processing enterprises. The scientific usage of feathers as a renewable material offers both economic and environmental benefits. The adsorbing ability of chicken feathers (CF) as potential biosorbent for the removal of hazardous substances from effluents is due to their high surface area and several reactive functional groups [8-11]. CF consists of keratinous proteins with many functional groups such as -COOH, -NH2, S-S- which can be treated with suitable chemical reagents to get chemically modified CF biosorbent.
The reagents used were ethanol, methanol and HCl and were of analytical grade. UV-Visible spectrophotometer (shimadzu-1800), Mechanical shaker (Rotek, Model number REC27255A2), pH meter, XRD (Rigaku Miniflex X-ray Diffractometer with Cu Kα radiation) etc were used.
Preparation of Biosorbent: The chicken feathers (CF) collected from poultry farms were cleaned, washed in water and ethanol and cut into pieces of size 5mm. CF was modified using an equimolar mixture of methanol and HCl for two hours. 10 g CF was mixed with 6% (v/v) CH3OH and 2% (v/v) HCl in a 250 ml double necked flask and placed on a hot plate at 80 0C with constant stirring for 3 hours. The reaction mixture was filtered, washed with distilled water and kept for drying [12]. Modified CF biosorbent was characterized by XRD & FTIR [13].
Preparation of Adsorbate: The stock solution of chromium metal ion was prepared in the range (1-10) × 10−5 M.
Batch Adsorption Experiments: The adsorption studies were carried out in batches in different conditions of pH, contact time, amount of adsorbent, temperature etc to check the propensity of adsorption process. In each 100 ml conical flasks, 25 ml of chromium solution was taken along with 100 mg of adsorbent and shaken for 2 hours in an orbital shaker which was then kept for 24 hours for saturation. Thereafter supernatant liquid was filtered through Whatmann Filter Paper No.42 and the amount of chromium ion adsorbed was determined spectrophotometrically at λmax 540 nm. The amount of chromium ion adsorbed per unit biosorbent (mg metal/g of biosorbent) was calculated using Equations (1) & (2)
Equation 1:
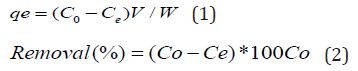
Where Co & Ce represent initial and final equilibrium concentrations (mg/L), V is the volume of Adsorbate taken, W is the weight of the biosorbent and qe is the amount of dye adsorbed at equilibrium.
Chemical modification of chicken feather was carried out with methanol as shown in Figure 1. Modified CF was characterized using XRD and FTIR. IR showed peaks in the range 1600-1700 cm-1 is due to -NH and -C=O stretching vibrations of the amide group. In the case of modified CF, this peak becomes sharp at 1653 cm-1 due to the formation of random coils at the expense of a α-helix and β-pleated sheets .The appearance of intense peak at 1740 cm-1 is due to the -C=O stretching vibration of the aliphatic ester of the modified CF [13]. XRD patterns of CF and modified CF are shown in Figure 2. The peak at 9.9 indicates α- helix configuration and a peak at 19 is due to stranded secondary structure. The modified CF peaks show decreased intensity. The slight shift of 2ϴ values confirms the decrease of the β-sheet content and partial cleavage of α- helix network [13] (Figures 1 & 2).
Figure 1: Chemical modification of chicken feather was carried out with methanol.
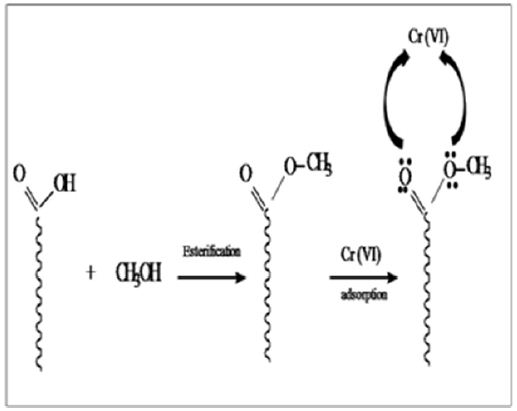
Figure 2: XRD patterns of CF and modified CF.
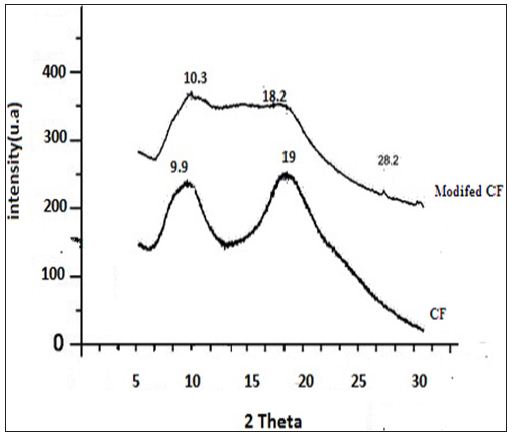
Figure 3 shows the variation of adsorption efficiency of CF with pH for chromium ions it was found that maximum adsorption occurs at pH 6.0. The effect of variation of contact time on the adsorption of metal solution was also studied and optimum time was found to be 2 hours as shown in Figure 4. From Figure 5 we can see that adsorption of the metal by modified CF also depends on the amount of sorbent used and the optimum amount was found to be 100 mg.
Figure 3: Variation of adsorption efficiency of CF with pH for chromium ions.
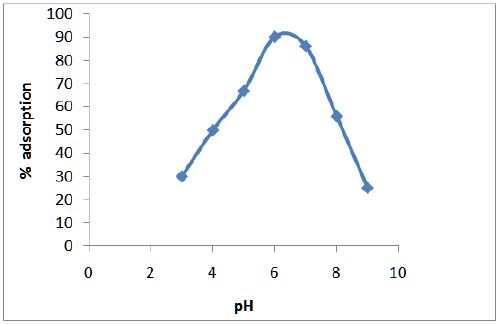
Figure 4: Effect of contact time on adsorption of matal.
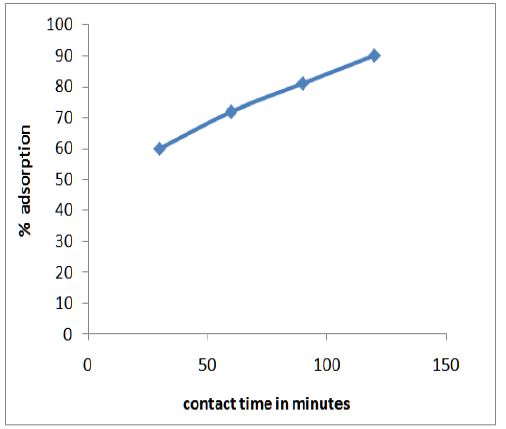
Figure 5: Optimum time was found to be 2 hours.
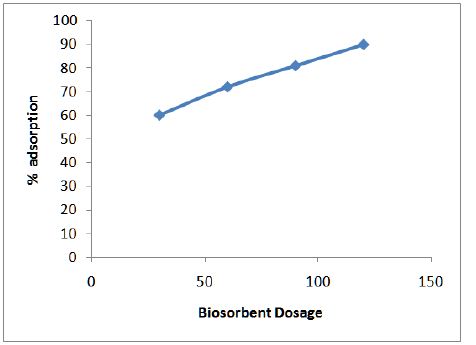
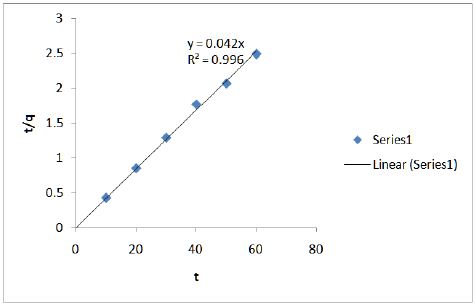
Figure 6 shows the effect of contact time on adsorption of chromium onto modified CF at 100 mg/L. Ho’s pseudo-second order model (Eq. 3) was used.
Figure 6: Effect of contact time on adsorption of chromium onto modified CF at 100 mg/L.
Equation 2:
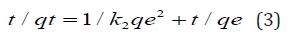
Kinetic studies show that the data fits well in the pseudosecond order plot and is shown in Figure 7.
Figure 7: Kinetic studies show that the data fits well in the pseudo-second order plot.
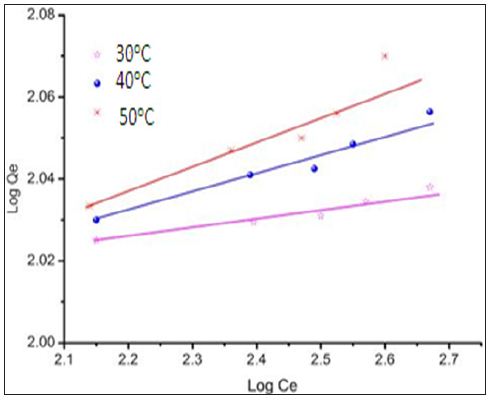
The Freundlich adsorption isotherm was applied for the adsorption of metal on modified CF.
The Freundlich equation is represented as:
Equation 3:
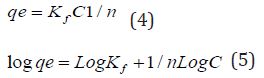
Figure 7 is the plot of equilibrium isotherm for the sorption of chromium ions on chemically modified CF. The data fits well in the Freundlich isotherm. It also shows the dependence of temperature on the adsorption of chromium ions on the sorbent which confirms the endothermic nature of biosorption.
This study showed that modified chicken feather could be used as a potential biosorbent for the removal of Cr (VI) ions from aqueous solution. The biosorption process was affected by contact time, temperature, pH and biosorbent dosage. The thermodynamic studies indicated the endothermic nature of the biosorption process. The Freundlich isotherm model was found to be the most suitable in describing the equilibrium of the biosorption process. The kinetics of adsorption followed pseudo-second order model.


