Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Devanand Mangar, Garrett Enten and Enrico Camporesi*
Received: November 03, 2017; Published: November 13, 2017
Corresponding author: Enrico Camporesi, TEAM Health Research Institute, 1 Tampa general Circle Suite A327, Tampa, FL 33606
DOI: 10.26717/BJSTR.2017.01.000520
Albumin has been used for fluid resuscitation in the OR and ICU, since 1940 [1]. Its usage gained prominence based on the classic descriptions of transvascular exchange by Earnest Starling who purported that colloids such as albumin should be more effective at increasing depleted intravascular volume due to their relative vascular membrane impermeability when compared to crystalloids such as saline [2]. It was not until 1998 that a systematic review by the Cochrane Injury Group Albumin Reviewers that the use of albumin for fluid resuscitation came under scrutiny [3]. In this first summary they described a 6 percent increase in mortality (relative risk 1.68, 95% confidence interval 1.28 to 2.23) in patients with hypovolemia, burns, and hypo-albuminemia who received albumin versus other fluids. This scrutiny lead to the landmark Saline versus Albumin Fluid Evaluation (SAFE) study published in the New England Journal of Medicine [4].
The SAFE study was a double blind randomized controlled trial that compared 4 percent albumin to 0.9 percent saline for fluid resuscitation in the ICU for a population of 6997 patients assigned to receive albumin or normal saline. This landmark study showed that when measuring primary outcomes over a 28-day period, albumin had no inherent advantages over saline in terms of mortality, length of stay, dialysis requirement, and mechanical ventilation duration. Moreover, The SAFE study investigators showed with subpopulation analysis that colloid fluid resuscitation was associated with a 19.6 percent increase in mortality in patients suffering from traumatic brain injury (relative risk, 1.88; 95% CI, 1.31 to 2.70; P<0.001)[5].
While critics of the SAFE study have concerns over the limitations of the 28-day observation period, they inevitably conclude the routine use of albumin for fluid resuscitation is not warranted for both the critically ill and for Intraoperative use [6,7]. Literature showing outcome associated benefits of albumin use is sparse and limited to specific patient subpopulations such as patients undergoing coronary artery bypass graft or suffering from septic shock [1,3,4,7,8]. However, these studies are limited in scope only measuring superficial physiological metrics such as hemodynamics or comparing albumin exclusively to other colloids [7,8].
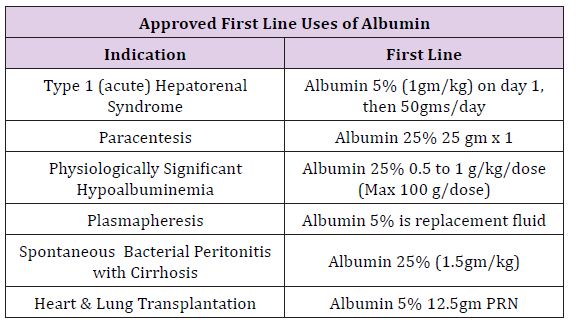
Tampa General Hospital is a large multispecialty facility of 1080 beds on the west coast of Florida, with major services in trauma, transplant, and specialty surgery for all ages. Average daily surgeries regularly exceed 200 patients in 60 staffing locations. After cost analysis and hospital-wide discussions, a memorandum accepted by the Pharmacy and Therapeutic Committee on September 2016 was circulated throughout the anesthesia department stating all forms of albumin would be removed from department endorsed anesthesia carts and all future requests for albumin were to be made at the pharmacy counter recording the physician name and indication. Following this memo, a series of guidelines were distributed department wide, to describe possible medical indications suggesting use of albumin and allowing for restricted distribution upon request by the Attending Anesthesia Provider. A synopsis of these Indications is described in (Table 1).
Table 1:
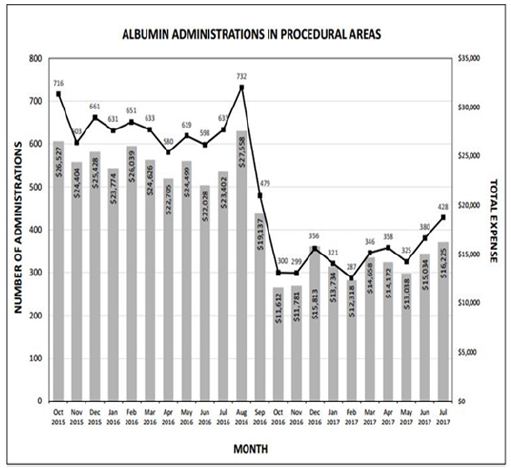
The rapid reduction in utilization was well received by providers in all anesthetic locations, as it was heralded and widely discussed weeks prior to implementation. Tracking of utilization and costper month are described in Figure 1, with a monthly summary and a 12 month follow up Table 2. Following restrictions on albumin administration, our institution was able to reduce average monthly albumin costs from $24,372 to $14,338 providing a potential annual savings of $120,000. After the first six months of the initiative, a slight increase in albumin utilization was noted resulting in a lower average monthly cost reduction than projected. This is possibly due to lack of continued monitoring and education, combined with employee turnover. However, this trend should be remedied with implementation of an education program for new employees and increased scrutiny for current employees to curb incorrect usage habits. Quality metrics such as in-hospital mortality, morbidity, and length of stay were not affected (Figure 1) and (Table 2).
Table 2:
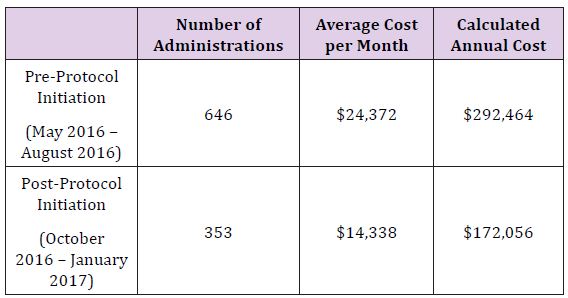
Figure 1:
With increasing manufacturing costs and lower production volumes, medical grade albumin has become very expensive. These cost increases in combination with the findings of the SAFE study have contributed to an increasing number of hospital initiatives to reduce albumin utilization for fluid resuscitation [7]. One initiative enacted by the University of Maryland Medical Center cardiac surgery intensive care unit was able to produce $45,000 worth of wholesale savings per month with no changes to morbidity or mortality. In this vein, implementation of similar albumin restrictive guidelines hospital wide would produce considerable CDF savings.
The based on the published data, the use of albumin in modern critical care medicine remains controversial. Although albumin supplementation for fluid resuscitation does not increase the relative risk of death or morbidity, it offers no measurable benefits when compared to crystalloids and other colloids. Following implementation of a restriction on albumin administration, our institution was able to reduce average monthly albumin administrations from 646 to 353, translating to a $10,034 decrease in monthly costs. On initial observation there was no difference in clinical outcomes.


