Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Azamris*1, Irwan1 and Hafni Bachtiar2
Received: October 30, 2017; Published: November 08, 2017
Corresponding author: Azamris, Cibinong Science Centre, Jl Raya Bogor Km. 46, Cibinong 16911, Indonesia
DOI: 10.26717/BJSTR.2017.01.000508
Breast cancer is a heterogenic disease with various biologic profiles and clinical prognosis. A research in Netherland by Esther, et al 2013, showed that molecular subtypes of breast cancer have different distribution and prognostic between young and adult, but there is no local and national data.A Comparative research with cross sectional design was Held in January-April 2015 with 96 samples of breast cancer women with age < 40 years old and > 40 years old, recorded in medical records and breast cancer registrated from 2012-2014. Young women Breast cancer at General Hospital Dr. M. Djamil Padang for 3 years (2012-2014) are 27.1% and the adult are 72.9%. In bivariate analysis, there are no relationship between characteristics of tumor with recurrent and death event while in the adult women breast cancer, tumor size and metastases have relation with recurrent and death event that are statistically significant. There are different characteristics and description of molecular subtype breast cancer between young and adult women, young ages are tend to have big tumor, lymph node positive, lympovascular invasion, high grade tumor, proliferation index Ki67 high and negative hormone receptor. There are different tendency of prognosis women breast cancer between young and adult based on molecular subtype but statistically, the relationship is not significant.
Keywords: Age; Breast Cancer Molecular Subtype; Prognosis
Breast cancer is a heterogenic disease with various biological profile and clinical prognosis [1]. Most patients with breast cancer are old women, but we still lack of specific guideline about evidence based therapy for this age group. Population of Young woman with breast cancer, otherwise, has based the decision about breast cancer therapy on prognostic, predictive factors, and tumor characteristic [2]. The European Society of Breast Cancer Specialist defined young women as women aged less than 40 years [3]. There is favorable correlation between ages with biological characteristic of the tumor. Compared with young age, old aged patient with breast cancer had more diploid, low s-phase fraction, normal p53, negative or low epidermal growth factor receptor and c-erB2 [4]. Breast cancer in women aged under 40 years old tend to has larger size (tumor median is 2 cm in young age and 1.5 cm in old age), more advanced stadium when diagnosed (more likely with positive lymph gland) and more aggressive (less likely to have good differentiation), low expression of estrogen/progesterone receptor (ER/PR), high expression of human epidermal growth factor receptor2 (HER2), and Ki67 marker proliferation [5-10]. Tumor with positive hormone receptor has better outcome, where luminal A tumor has slower progressivity compared with luminal B tumor. While tumor with negative hormone receptor have aggressive natural pathogenesis and poor outcome [2].
Although it has been given the optimal treatmentt, but some clinical trials showed that breast cancer patient with young age had worse outcome compared with old aged breast cancer patient [10]. The distribution and prognostic effect of certain molecular subtype from old aged breast cancer patient compared to young aged breast cancer patient is remains unclear. A study conducted by Esther et al. [10] in Netherland showed that breast cancer molecular subtype has distribution and prognostic effect difference between old aged breast cancer patient compared to young aged breast cancer patient. While the data about comparison about distribution and breast cancer molecular subtype prognostic between young and old aged breast cancer patient, either local or nationwide, was not found by author. This is why author wanted to investigate thedifference of breast cancer molecular subtype prognostic between young and old aged breast cancer patient at Dr. M DjamilGeneral Hospital, Padang.
Population and Sample: The population in this study was all of the breast cancer patient who registered at the medical record and breast cancer registration on Divison of Oncology Surgical Department Dr. M Djamil Hospital Padang.The sample in this study was breast cancer patient who diagnosed andtreatment according to protocol and registered atthe medical record and breast cancer registration on Divison of Oncology Surgical Department Dr. M Djamil Hospital Padang in 2012-2014 by using the simple random sampling technique.
Female breast cancer patients young age (≤ 40 years) and older (> 40 years) who had done histopathology and immunohisto chemistry examination. Exclusion criteria:
I. Breast cancer patients with cancer of the other organs were not metastasis of breast cancer
II. Breast cancer patients who died within a period of three years by another cause
III. Breast cancer patients with medical records could not be traced.
Figure 1 in this study, the data collected was secondary data from medical record archives and breast cancer registration in Division of Oncology Surgical Department Dr. M Djamil General Hospital Padang.
Figure 1:
The data in this study were been through several process including:
a) Data Editing: Conducted in the data storage to make sure that the data in medical record and breast cancer registration met the criteria so the mistake can be avoided
b) Data Coding: the data was given number and code to make the analysis easier
c) Data Entry: the data was inputted into the computer
d) Data Cleaning: to recheck if there was any mistake in data and also to correct and examine the data to make sure the data was valid
Research result was found after all of data from medical records and breast cancer registrations in Oncology Surgery Division of Surgery Department of Centre General Hospital Dr. M. Djamil Padang years 2012 - 2014. Home visiting was done for patients who live in West Sumatera Province. Patients that live out of West Sumatera be interviewed by phone or interviewed when they were following up to Oncoligy Surgery Polyclinic. The number of samples in this research was 96 breast cancer patients who have inclusion criteria. From 96 breast cancer patients, all of them had histopathological diagnosis, immunohistochemistry status, but only 32 patients had lympovascular invasion status and 59 patients had their histopathology grading. Data was processed computerize. Data analyses was done descriptively and using Chi - square test for finding correlation between 2 variables with confidence index was 95%.
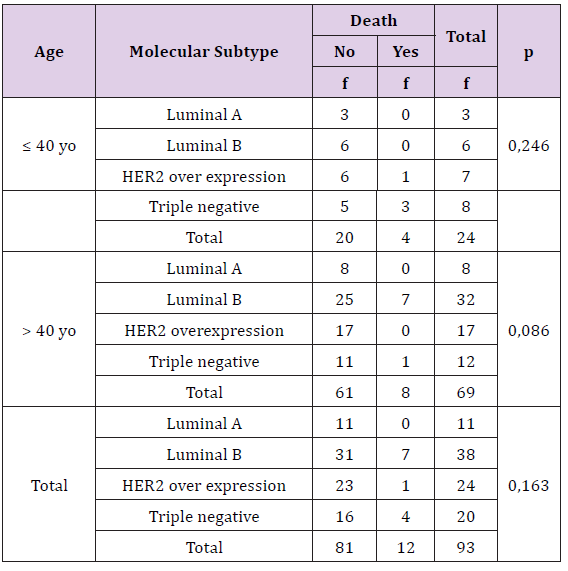
Table 1 from 15 death patients, 12 dead caused by breast cancer and 3 because of other causes.
Table 1: Distribution Frequency of Breast Cancer Characteristics.
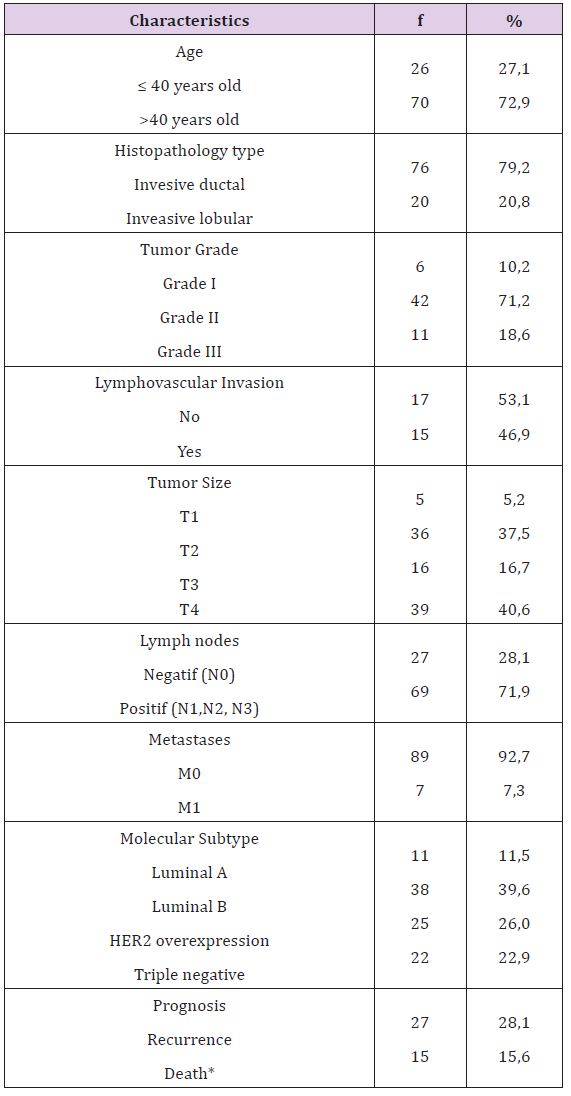
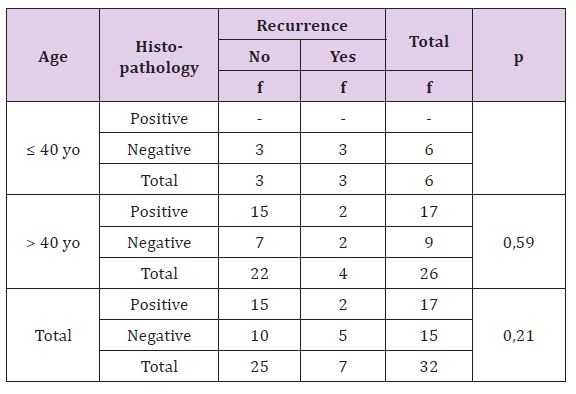
Table 2 statistically, this difference is not significant (p>0,05), there is no correlation between histopathology type of breast cancer with recurrence rate both age or either overall criteria (Table 3).
Table 2: The Correlation of Histopathology with Result of Recurrence Treatment Based on Patient’s Age.
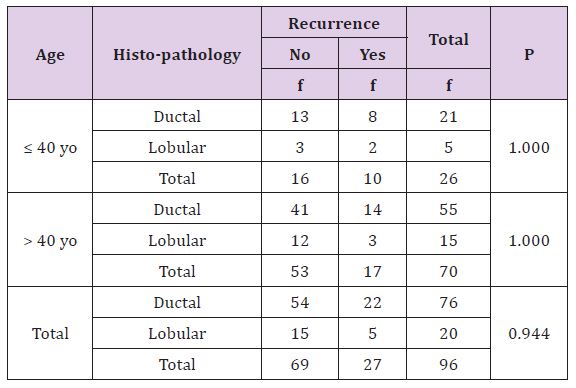
Table 3: The Correlation of Histo-pathology with Death Rate Based on Patient’s Age.
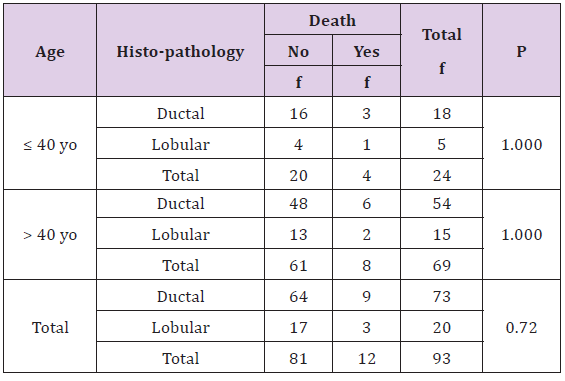
Statistically, this difference is not significant (p > 0, 05), there is no correlation between histopathology type of breast cancer with death rate both age or either overall criteria. The percentage of death is more in patient with invasive lobular carcinoma type.
Table 4 statistically, this difference is not significant (p >0, 05), there is no correlation between tumor grade with recurrent both age or either overall criteria. The young age has more recurrence rate than adult patient, especially in grade III tumor (Table 5). Statistically, this difference is not significant (p >0, 05), there is no correlation between tumor grade with death rate based on age or either other all criteria. Based on tumor grade, young breast cancer patient’s survival rate is less bad (Table6).
Table 4: The Correlation of Tumour’s Stage with Result of Recurrence Treatment Based on Patient’s Age.
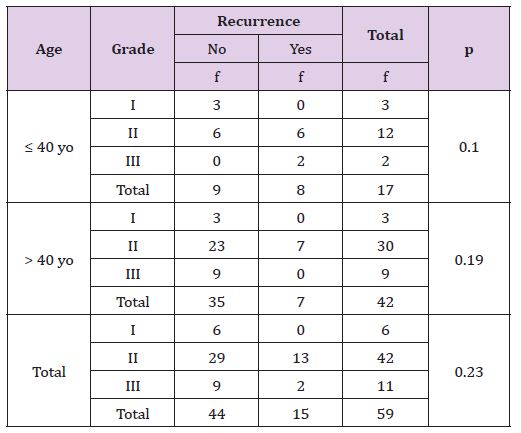
Table 5: The Correlation of Tumour’s Stage with Death Rate Based on Patient’s Age.
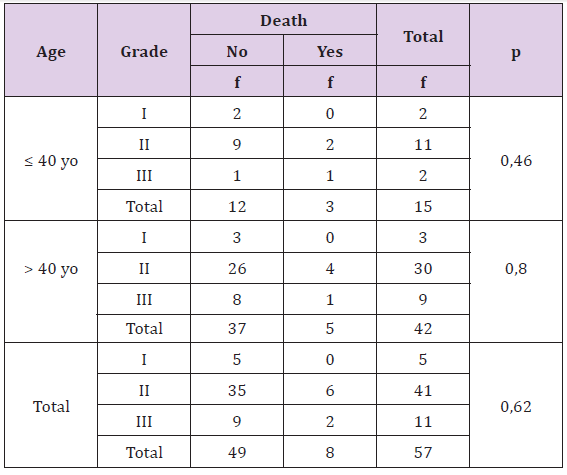
Table 6: The Correlation of Lymphovascular Invasion with Result of Recurrence Treatment Based on Patient’s Age.
Statistically, this difference is not significant (p >0, 05), there is no correlation between lympovascular invasion with recurrence rate both age or either overall criteria. Statistic test could not be done in young age because of no recurrent data in patient without lympovascular invasion. Recurrent percentage is more in breast cancer patients with lympovascular invasion (Table7). Statistically, this difference is not significant (p > 0, 05), there is no correlation between lymphovascular invasion with death rate in breast cancer patient. Statistic test could not be done in young and adult because of there is no enough data. Generally, more of breast cancer patients died with lympovascular invasion (Table 8).
Table 7: The Correlation of Lymphovascular Invasion with Death Rate Based on Patient’s Age.
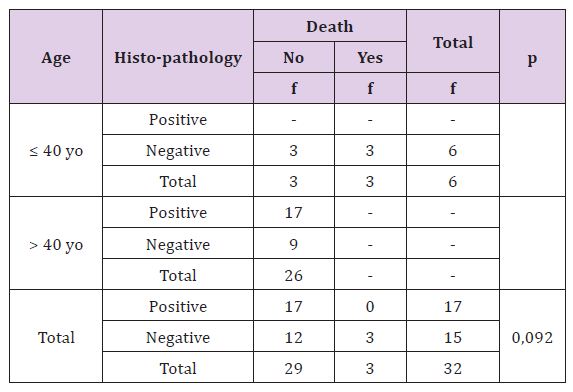
Table 8: The Correlation of Tumor Size with Result of Recurrence Treatment Based on Patient’s Age.
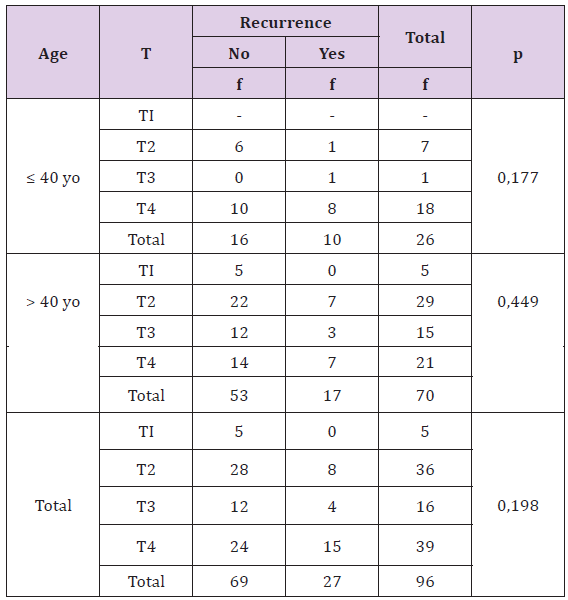
Statistically, this difference is not significant (p >0, 05), there is no correlation between tumor size with recurrence rate, although based on age or other all finding. Young breast cancer patients have bigger size and more recurrence rate compared with adult patients (Table9). Statistically, this difference is significant (p <0, 05), there is correlation between tumor size with death rate, but not significant on younger age. Young breast cancer patients have higher death rate than adult patients, and the young has bigger tumor size (Table10).
Table 9: The Correlation of Tumor Size with Death Rate Based on Patient’s Age
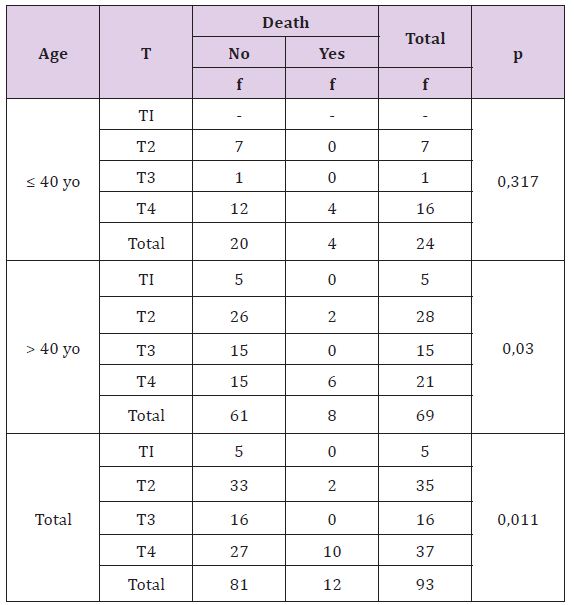
Table 10: The Correlation of Glands Lymph Involvement with Result of Recurrence Treatment Based on Patient’s Age.
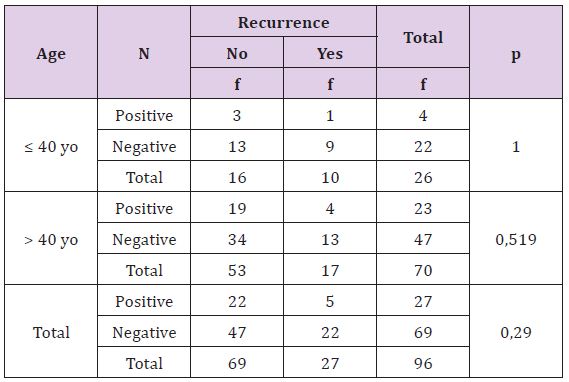
Statistically, this difference is not significant (p >0, 05), there is no correlation between glands lymph status with recurrence rate based on both age or either overall criteria. Young breast cancer patients with positive glands lymph involvement have higher recurrence rate than adult patients (Table11). Statistically, this difference is not significant (p >0, 05), there is no correlation between glands lymph status with death rate on breast cancer patient, both age or all other. Young breast cancer patients with positive lymph glands have higher death incidents than adult patients.
Table 11: The Correlation of Glands Lymph Involvement with Death Rate Based on Patient’s Age.
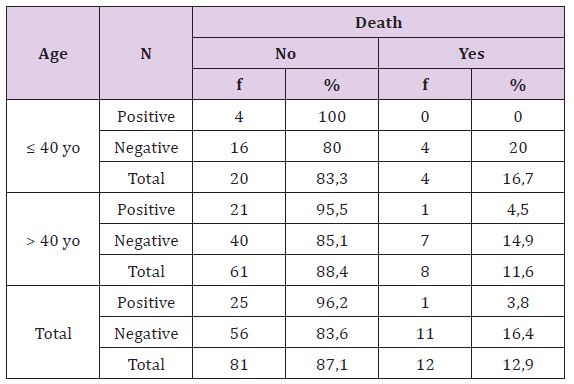
Table 12 statistically, this difference is significant (p <0, 05), there is correlation between metastasis with death on adult patients and overall, but not significant on young patients. Young breast cancer patients without metastasis are more death than adult patients.
Table 12: The Correlation of Metastasis with Death Rate Based on Patient’s Age.
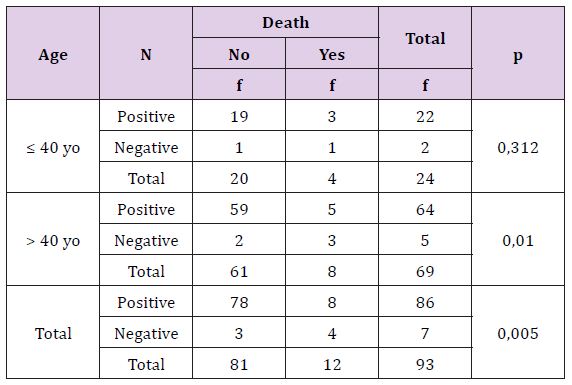
Table 13 statistically, this difference is not significant (p >0, 05), there is no correlation between breast cancer’s molecular subtype with recurrence based on age either overall. Young breast cancer patients with any molecular subtypes are more recurrence than adult patients (Table 14). Statistically, this difference is not significant (p >0, 05), there is no correlation between breast cancer’s molecular subtype with death based on age either overall criteria. The death breast cancer patients are more on young age, especially triple negative subtype.
Table 13: The Correlation of Molecular Subtype with Result of Recurrence Treatment Based on Patient’s Age.
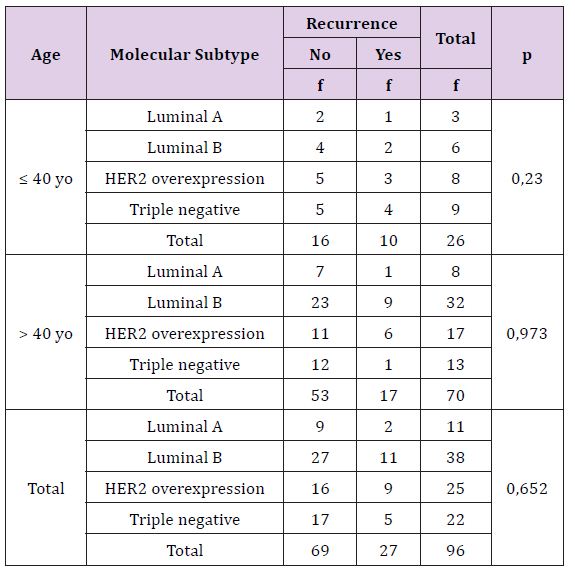
Table 14: The Correlation of Molecular Subtype with Death Rate Based on Patient’s Age.
From medical records data and breast cancer registry, there were 96 breast cancer patient who underwent treatment in dr. M. Djamil General Hospital from 2012 until 2015. The difficulty in this study was the lack of evaluation of tumor’s grading and lymphvascular invansion’s status by anatomy pathology department, there were only 59 patients of 96 patients who had tumor grading and only 32 patients who had lymphovascular invansion’s status. There were 27 cases of recurrent breast cancer and 15 patients died, but only 12 patients who died of breast cancer.There were many factors affect the prognostic of breast cancer and age was the most important factor for recurency risk whithout depend on the other factors (indenpendent risk factor) [11-14]. There was relationship between age and biological characteristic of tumor [4]. Patologic subtype is one of prognostic factors breast cancer. The most histopathologic type of breast cancer was invasive ductal carcinoma [15]. There was a differency between invasive ductal carcinoma and invasive lobular carcinoma. Invasive lobular carcinoma was more occured with old age, biggest tumor size, positive of receptor hormone, negative of HER2 and p53, and rarely invade the vascular if compared with invasive ductal carcinoma, but it often multifocal, multicentric and bilateral, associated with increased risk of contralateral breast cancer, low grade histology, often metastasize to unusual locations like gastrointestinal tract and died. Particulary, invasive lobular carcinoma show characteristics that lead to good prognostic compared to invasive ductal carcinoma [16,17].
In this study, invasive ductal carcinoma was the most type occured which is 76 patients (79,2%). From 20 invasive lobular carcinoma’s patients, most of them occured at old age (> 40 years old) which were 15 patients. However, percentage of reccurent events and death was greater in young cancer patients. (38,5% and 16,7%) with same type histopatology which was invasive lobular carcinoma (40% and 20%). If it associated with other tumor characterisics, invasive lobular carsinoma in young age had tumor with T4 stadium and positive lymph node. Most of them had ER (-), PR (-), and HER2 (-). One of patients had lung metastasis. In this study showed that characteristic tumor was more influenced by young age than other histopathologic tumor although invasive lobular carcinoma had better prognostic, it will be a bad prognostic if it occured in young age. Grade of histology tumor assessed by differentiation degree of tumor tissues.50 Tumor with high grade had abnormal shape and tend to aggressive, reccurency, and metastasize. Breast cancer patients with high grade tumor include in the high risk group and indicated to had adjuvant chemotherapy. There was reverse relationship between tumor differentiation grade with pathomorphological response (PMR) grade in breast cancer patients who had neoadjuvant chemotherapy, where well differentiated tumor had bad respon.
In this study, recurrence and death was higher than tumor with grade II and III. Based on age, young patient had worse prognostic than old patient because most of them had grade III tumor. In old patient, the recurrence and death more frequent in grade II tumor. In general, these tumor had big size (T2), had positive lymph node when diagnosed, vary response of hormone receptor but most of them had high index of Ki6714%. This was concordant with characteristic of high grade tumor, which was the higher tumor’s grading means more aggresive and fast proliferations that noted by high index of Ki67, bigger size, and invasion to other tissue like lymph node but there was no far metastasis when diagnosed. Most of these patients had adjuvant therapy, but there were recurrence, both locoregional or far, and died in 3 years after diagnosed. This was caused by reccurency process occured when treatment and other criteria like young age and positive lymph node caused patients in high risk group. Invasion of lymphvascular was an important step in complex process for metastasis and an important criteria to decide the next treatment [18]. Invasion of lymphvascular was an indenpendent bad prognostic factor on invansive breast cancer patients [19].
Young Ju Song et al. reported percentage of OS and DFS 5 years lowest in patients with lymphvascular invasion compared whose not. In this study, patients with lymphvascular invasion had bad prognostic with higher percentage of recurrence and death (33,3% and 20%). The result of statistic test showed there was no relationship between lymphvascular invasion with residif and death (p+ 0,209 and p= 0, 092). There was no data about recurrence in young patients without lymphvascular invasion, it was difficult to compare prognosis between these both group age with lymphvascular invasion. This was caused by the lack of data, there only 32 patients of 96 patients that had lymphvascular invasion status. Lymphovascular invasion is a step in complex process of tumor metastasis and also important for the next treatment. Lymphovascular invasion is a poor prognostic factor which is independent in patient with invasive breast cancer. Young Ju Song, et al has the percentage of OS and DFS 5 years lower in patient with lympovascular invasion compared with the patient without lympovascular invasion. In this research, patient with lympovascular invasion have a poor prognostic with percentage of recurrence and mortality (33,3% and 20%). Statistical test result shows there is no connection between lympovascular invasion with recurrence or mortality (p = 0,209 dan p = 0,092). There is no data or evidence about recurrence condition in younger patient and mortality in older patient with or without lympovascular invasion, so it is hard to compare the prognosis from age category. This is due to only 32 out of 96 patients that have lympovascular invasion.
All of the patients with lympovascular invasion with recurrence condition or death have histopathological type of ductal invasive carcinoma, this is in accordance with the literature that said this type of histopathological more often invade the lympovascular tissue compared to invasive lobular carcinoma. Young patient have worse prognosis. Every young patient with recurrence condition, died after treatment, but in the other hand there is no mortality in old patient with recurrence condition. Characteristic of tumor in young patient has poor prognosis due to a large tumor size, presence of positive lymph node, high grade tumor, negative hormone receptor and also high proliferation index of Ki67. There is accumulation from several poor prognostic factors in young age patient with breast cancer. The difference is, old patient with breast cancer have better prognosis due to presence of positive estrogen and progesterone also low grade of Ki67. Tumor size is one of prognostic factor in breast cancer [13]. Tumor size is correlated to the presence and amount of axillary lymph nodes involved, and also an independent prognostic factor, with increase recurrence that is concomitant with tumor enlargement [20]. Lethality of breast cancer increase with tumor size and presence of regional lymph node [21].
In this study, the incidence of recurrence and mortality increase with tumor size (38,5% and 27%). Young patient with breast cancer tend to have bigger size of tumor and higher recurrent incident compared to old patient with breast cancer. Percentage of tumor with T4 in young age patient who experience recurrent (44, 4%) while in old patient (33,3%). Most of tumor with T4 has positive lymph nodes in both ages. Young patient with breast cancer has more mortality with percentage (16, 7%) compared to old patient (11,6%), however tumor with T4 has more mortality in old patient (28,6%). This is likely due to most of patients already having distant metastasis when diagnosed. This correlation was statistically significant in tumor size with mortality incident in old patient (p = 0, 03) or mortality incident in old patient with metastasis (p = 0,01). This can be explained that most mortality of death patient with T4 tumor has metastasis. Hormone receptors, expression of HER2 and Ki67 are the prognostic factor of breast cancer. Tumor with positive hormone receptor have better outcome compare to tumor with negative hormone receptor. Ki67 describes the proliferation of tumor cell and provide information and independent prediction of the response to chemotherapy and prognosis for breast cancer patient who received neo adjuvant chemotherapy [22,23]. Breast cancers in young woman (< 40 years old) tend to have low estrogen or progesterone expression and high expression of HER2 also proliferation marker of Ki67. The result of this study in accordance with literature that young woman with breast cancer (< 40 years old) has worse prognosis than older age (>40 years old) with higher recurrent percentage and mortality (38, 5% vs 24,3% dan 16,7% vs 11,6%).This poor prognosis at young age also in accordance with molecular subtypes that is triple negative (ER/ PR -, HER2 -). Recurrent occurrence at old patient (>40 years old) is more common in over expression of HER2 subtype and higher percentage of mortality in luminal B subtype.
However statistically, correlations between molecular subtypes with the prognosis of breast cancer based on age were not significant. Recurrent incident and mortality in younger patient is higher than older patient in every molecular subtype, this is probably because accumulation of poor prognosis factors based on age group which is large tumor size, positive lymph nodes, presence of lympovascular invasion, high grade tumor, high index of Ki67 proliferation. That means young age is associated with poor prognosis of breast cancer. Like other cancer, breast cancer is considered as part of the accumulation of multiple genetic changes resulting in excessive expression of oncogenes and loss of tumor suppressor. DNA methylation changes will ultimately lead to instability in genetic characteristics of cancer through various ways [24-26]. Data of methylation has been associated with clinico pathologic parameter to clarify the role of methylation in breast cancer carcinogenesis. Report from Widschwendter, et al showed a significant difference in hormone receptor status among the group with DNA methylation [27-30]. Through Southern analysis of BRCA1 promoter region, methylation was found in 11% sporadic breast cancer cases and inversely related to the expression of estrogen and progesterone receptors [24]. Song ping et al assessing the relative frequency of methylation in the two groups based on age between African-American and European-American. They found that young age (< 50 years old) breast cancer patient ethnic African-American and negative ER had significantly higher index of methylation in CDH13 locus compared to breast cancer patients ethnic European-American with the same characteristic [31-40].


