Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ezgi Aydın* and Hasan Yeler
Received: October 25, 2017; Published: October 30, 2017
Corresponding author: Ezgi Aydın, Cumhuriyet University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, 58140, Sivas, Turkey
DOI: 10.26717/BJSTR.2017.01.000477
Purpose: Osseointegration is the structural and functional connection without fibrous tissue, between the bone and the implant surface under load. Free radicals are among the factors that affect bone healing and osseointegration. Recently, researchers have begun to show interest in the idea that implant-related complications may occur due to free radical-induced tissue damage. Hence use of a material with antioxidant properties may be a useful tool on implantology. This study is planned presuming propolis could have positive effects on osseointegration and implant stability due to its antioxidant nature, its effect accelerating the bone formation, wound healing and increasing the bone density.
Methods: Study consisted of three groups. At all groups, implants were placed on proximal metaphyseal region of the rabbit tibia. Only dental implants were placed on the Control Group. Propolis solution was applied to slots before placing the implants on Local Group and propolis was applied systemically for 28 days after implantation on Systemic Group. Resonance frequency analysis (RFA) was performed on the day implants are placed and both RFA and removal torque test were performed on the 28th day.
Results: The study was composed of 24 rabbits grouped as follows: Control (n = 8), local (n = 8), and systemic (n = 8) for application of propolis. Systemic group showed statistically lower results of RFA at baseline (p<0.05). Propolis application resulted in a significant increase in RFA in each group: local (baseline RFA, 46,8 vs RFA after Propolis 56.0; P< 0.05), systemic (baseline RFA, 44.8 vs RFA after propolis, 58.8, P< 0,05); while the control group showed no significant difference (baseline RFA, 51.5 vs RFA after waiting period, 57.8 P> 0.05). Removal torque test showed statistically significant difference between the groups (systemic group 58,7; local, 49,8 and control, 45,1; P<0.05).
Conclusion: These results suggest that the propolis may have a positive effect on implant stability, especially if administered systemically.
Propolis is a natural product bees prepare by enzymatically changing secretions they gathered from the buds and barks of plants. Nowadays, propolis has syrup, spray, throat lozenge, cream and lotion preparations which are formulated to prevent and treat upper respiratory tract infections like cold and flu, for use in cosmetic purposes and in dermatological problems such as wounds and burns, also infections like acne, Herpes Simplex 1 and 2. In addition, there are toothpaste and mouthwash preparations used in the caries prevention and treatment of oral infections such as gingivitis and stomatitis [1]. Investigations have shown that propolis has antimicrobial, antiviral, antifungal, antiprotozoal, antiparasitic, antiinflammatory, anti-ulcer antitumor and antioxidant effects and protective effects on liver, heart and brain [2].
Propolis has been shown to regulate the blood glucose level and the blood pressure, to affect the amount of lipid in blood and to cleanse lipid peroxidation products and free radicals [3]. In addition, propolis has immunostimulant, antimutagenic [4], anesthetic [5,6] and analgesic [7] effects. Osseointegration is the structural and functional connection without fibrous tissue, between the bone and the implant surface under load [8]. There are many factors that affect osseointegration. Primer stability, implant surface characteristics, anatomical state of implant area, prosthesis design, occlusion pattern on healing phase and bone metabolism constitute some of these factors [9]. Free radicals are among the factors that affect bone healing and osseointegration. Recently, researchers started to show interest in the idea that implantrelated complications may occur due to free radical-induced tissue damage [9-10].
On healing process after implantation, granulation tissue is minimal and well-organized bone formation is observed [11]. Following the placement of the implant, with the necrosis and resorption of the traumatized bone, new bone formation and a series of healing events occur in the area around the titanium body [11,12]. Following the implantation, the amount of contact between the implant and the surrounding bone increases due to bone healing. Treatment may fail if implants are loaded before this contact reaches a certain level. For this reason, loading time should be decided based on the implant stability [13].
Based on the literature review, we thought that propolis may shorten the consolidation phase after implantation and have a positive impact on implant stability. In this study, we aimed to investigate the effects of propolis on stability and to help to develop new agents that will support the osseointegration and bone healing process.
This study was approved by the Cumhuriyet University IRB and the animal care and use committee.
For our study, 24 male New Zealand rabbits (Oryctolagus Cunilus L), approximately 6 months old and weighing about 2.5- 3 kg, were obtained from Cumhuriyet University Experimental Animals. Food and water requirements of all animals were met without limit. Standard conditions were applied in the animal room (22-24 °C, 55-70% humidity, 1 atm, 12 hours light / dark room). During the study period, animals were placed in stainless steel cages measuring 50 x 80 x 50 cm, with each cage containing a single rabbit. All animals were brought to the laboratory one month prior to surgical procedure and monitored to ensure optimal health conditions, protection from infections, and adaptation to their new location. Animals were divided into 3 groups, each consisting of 8 rabbits.
Animals went under anesthesia by intramuscular injection of 10-20 mg / kg Xylazine (Rompun 2%, Bayer, Istanbul, Turkey) and 50 mg / kg Ketamine HCl (Ketalar, Eczacıbaşı-Warner Lambert, İstanbul, Turkey) before the surgical procedure. 3.5 x 10 mm sized dental implants with external hexagonal platform, were obtained from ADIN Dental Implant Systems (SLA Surface, Toureg-NP, Afula, Israel). The unilateral tibia proximal region was shaved and cleaned with antiseptic solution on each rabbit. Surgical area was prepared by coating with sterile surgical drapes and sterile film. A 2-3 cm incision was made from the proximal to distal metaphyseal region of the tibia. Subcutaneous tissues and muscle layers were dissected with blunt dissection to reach tibia surface. Implant slots were prepared using burs at the rate of 600-1000 rpm, under sufficient isotonic solution irrigation, according to the company’s recommendations (Table 1).
Table 1: Difference of surgical procedures on groups.

Dental implants were placed in the slots using a ratchet and attention was paid to have adequate primary stabilization. After implantation, RFA measurements were made with Ostell (Integration Diagnostics AB, Göteborg, and Sweeden). After the operation, elevated epidermal flap was sutured to its original position with 5-0 polyglactine 910 (Vikril Jonson & Johnson / Ethicon) attaching the muscle, subcutaneous fascia and skin. Postoperatively, 50 mg / kg Ceftaxon (Cephaxon-Toprak) IM and 4 mg / kg Carprofen (Rimadyl-Pfizer) were administered subcutaneously for 3 days. 200 mg / kg / day of the propolis was given to the rabbits in Systemic Group by oral gavage for 28 days. All the rabbits were taken to a 28-day rest period under pre-prepared, appropriate ambient conditions. At the end of the 28-day follow-up period, animals were sacrificed via 200 mg / kg i.p. sodium pentobarbital injection.
1g of propolis (Eğriçayır Organic Bee Products / Mersin) was added to 1 ml dimethyl sulfoxide (DMSO) and vortexed for 1 minute (Jeio Tech, Vortex Mixer VM-96T). The prepared mixture was put in an ultrasonic bath (Kudos HP Heating Series ultrasonic cleaner) and stirred at 25 °C and 53 kHz, for 15 minutes. After filtering via sterile filters with 0.22 μm pore size, prepared solution was diluted with saline (1: 4; DMSO: saline) and got ready to use.
Osstell® was used to measure the resonance frequency. The measurements were made by placing the Smart Peg on implants. After inserting the implants and at the end of follow-up period, resonance frequency analysis was performed and the procedure was repeated 5 times for each implant. At each measurement, the most repeated result was accepted as correct.
All rabbits were subjected to removal torque test after RFA measurement at the end of follow-up period. The probe of the digital torque meter (Lutron TQ-8800; Lutron Electronics Enterprise, Taipei, Taiwan) was attached to the implant, and force was applied in a controlled and increasing way reverse clockwise. The moment implant began to rotate within its slot, force was stopped. Highest value on the digital display was recorded in N / cm.
The data obtained from our study were uploaded to the SPSS (ver: 22.0) program and evaluated. When the parametric test assumptions are fulfilled (Kolmogorov-Simirnov) Variance Analysis and Tukey Test; when the parametric test assumptions were not met, the Kruskal - Wallis Test was applied and the P-values was taken as 0.05.
One of the animals in control group died due to diarrhea. None of the other subjects had a pathology caused by the device or method that affected their general health. Findings in the study were analyzed in two parts as resonance frequency analysis and removal torque test. Measurements were performed separately foreach group (Figure 1). When the operation day measurements of resonance frequency analysis in all three groups were compared, the difference was significant (P <0.05). When the measurements were compared in pairs, difference between the control group and the systemic group was significant, but no significant difference was found between the control group and the local group or the local group and the systemic group (P> 0.05). When the resonance frequency measurements were compared on the 28th day in all three groups, the difference between the groups was not significant (P> 0.05) (Figure 2).
Figure 1: Distribution of RFA 1st day values of groups.
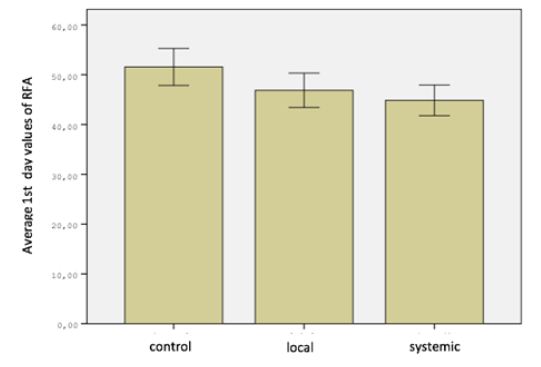
Figure 2: Distribution of RFA 28th day values of groups.
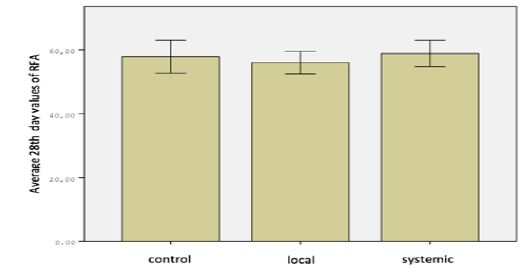
Table 2: Comparison of RFA 1st day and 28th day differences between groups.
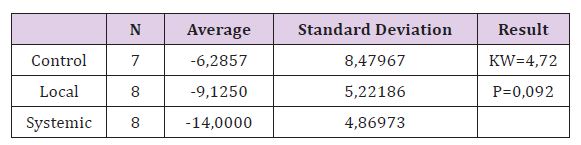
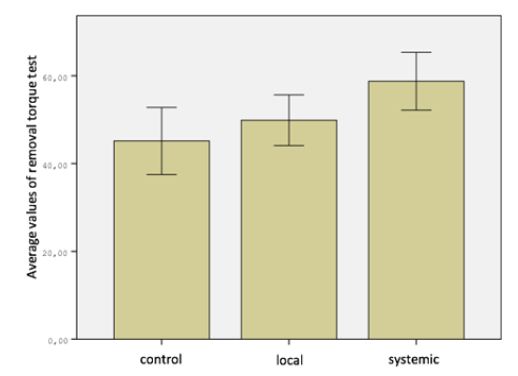
When comparing the removal torque test measurements in all three groups, the difference was significant (P <0.05). When the removal torque test measurements were compared in pairs difference between the control group and the systemic group was found to be significant, but no significant difference was found between the control group and the local group, the local group and the systemic group (P> 0.05) (Figure 3). There was no significant difference between the RFA measurements of Control Group on day 1 and day 28 (P> 0.05). In the Local Group and Systemic Group, the differences were significant when comparing RFA 1st and 28th day measurements (P <0.05). The most important difference is seen in the systemic group (Table 2). When improvement of RFA from 1st to 28th day in all three groups was compared, the difference between the groups was found to be statistically insignificant. But the biggest difference is seen in the systemic group. This result may be due to the low number of subjects.
Figure 3: Distribution of RFA 28th day values of groups.
There are defensive mechanisms in the organism which prevent reactive oxygen species from forming and damaging them. These are called antioxidant defense systems or antioxidants. Studies show that antioxidants play a protective role against free radicals [14]. Propolis is a very complex natural compound that has been in use since ancient times for treatment and is still in use today. In-vitro antioxidant activity of propolis has been shown in many studies conducted with different methods. Benzyl caffeate, the high amount of phenolic component and caffeic acid phenyl ester which is the active component of propolis has been suggested to be responsible for its antioxidant activity [15].
Propolis has antibacterial, anti-inflammatory, antitumor, analgesic and anesthetic effects [15,16]. Anti-inflammatory, hepatoprotective, cardio protective and radio protective effects of propolis are associated with its high antioxidant activity [17]. There are many studies investigating the effects of propolis on bone. In a study by Altan et al., effect of systemically applied propolis on the expanded premaxillary suture was studied and it was thought that bone formation in this region could be accelerated [18]. In a study by Guney et al., effects of propolis on fracture healing were examined and the time-dependent, beneficial effects of propolis were discussed. Toker et al. [19] investigated the effects of systemic propolis on morphometric and histopathological changes in experimental periodontitis induced in rats and found propolis to prevent alveolar bone loss [20].
Based on these studies, we suggest that the propolis may shorten the consolidation phase after implantation and have a positive effect on implant stability. In addition, due to its antiinflammatory and anesthetic effect, propolis may reduce postoperative stress. There is no standard for the extraction procedure or thecomposition of propolis-containing extracts [21]. Traditionally, the dissolved fraction of propolis in 70% ethanol is extracted and is called propolis balsam [22]. However, DMSO has been shown to be less toxic than ethanol [23,24]. In a study investigating the antimicrobial activity of propolis, DMSO extracts showed higher antimicrobial activity than acetone extracts [25]. In another study using DMSO, experimental periodontitis was induced in rats and systemic propolis administration decreased alveolar bone loss [20]. Taking these studies into consideration, we decided to use DMSO in the preparation of the propolis solution.
An official dose limit for propolis has not been established. However, in rats LD50 was found to be 2-7.34 g / kg. The recommended daily dose is 1.4 mg / kg [26,27]. Studies have indicated that propolis is toxic when administered over a concentration of 2000 mg / kg [16]. In a study on rats, propolis administered via oral gavage at 500 mg / kg / day following intraperitoneal implant placement was found to have mitigating effects on inflammation, angiogenesis and fibrogenesis [27]. In a study conducted by Bereket et al., effect of propolis on the consolidation phase was investigated after distraction osteogenesis was performed on rabbits and the applications at oral doses of 100 mg / kg / day and 200 mg / kg / day were compared. It was observed that the 200 mg application was more effective in accelerating new bone formation [28]. We did not encounter any experimental or clinical study investigating the effect of propolis on implant stability. Therefore, the duration, dosage and regimen for propolis after implantation was determined for the first time in this study but studies investigating effects of propolis on bone healing constitutes a basis for our research. Therefore, systemic propolis administration course was selected as 200 mg / kg / day.
There are many studies in which propolis is applied locally. In a rabbit study, the intraarticular administration of propolis in the experimental septic arthritis model yielded beneficial results [29]. Another study showed that a layer of propolis application with allograft enhanced and accelerated osseointegration and shortened the consolidation phase [30]. In addition, the ability to obtain a dense form of propolis that is homogeneously dissolved, has no toxic effect and can be easily applied to the implant cavity, allowed us to plan the study in this way. During the experiment, the surgical procedure and placement of implants were performed by a single operator, aiming to standardize.
The selection of rabbits as experimental animals in our study is due to the fact that physiological bone healing in rabbits is similar to humans, and at the same time the rate of healing is three times higher than that of humans [31]. Physiologically, an 8-week healing period in rabbits is equal of 6 to 8 months of healing in humans [32]. The 4-week recovery period for rabbits is thought to be sufficient for monitoring new bone formation and angiogenesis [33]. Therefore, we opted to perform the measurements on the 28th day to achieve appropriate osseointegration, bone healing and implant stability. One of the major problems faced by dentists is the assessment of the osseointegration of the dental implant. The amount of osseointegration between the implant surface and the bone supporting the implant is used to measure the stability of the implant [34]. This measurement can be made with many different techniques [34,35]. Most important methods are percussion test, Periotest, resonance frequency analysis and removal torque test.
The use of percussion sounds to measure implant stability is a highly subjective method and is not reliable enough. One of the first devices used to provide an objective stability measurement was the Periotest, initially designed for measuring the stability of the teeth, but also for implant stability [36,37]. However, reproducibility of the results obtained with Periotest is affected by many factors such as the vertical position of the measuring point on the abutment, the angulations, and the distance between the hand piece and abutment [38]. The removal torque test is one of the in vivo mechanical tests used to evaluate the strength of the connection between the bone and the implant surface [31,39,40]. When removal torque is tested, it is assumed that the implant’s high resistance to the extraction indicates that there is a high amount of bone formation and contact between the bone and the implant surface. Ivanoff et al. [40] have shown that the removal torque value is closely related to the bone implant contact and the amount of bone entering the grooves.
They reported that the need for higher torque forces to remove implants can be interpreted as an increase in bone implant contact and an increase in osseointegration strength [41]. However, removal torque test may not be the most effective method for measuring the amount of bone around the implant or the fixation. The underlying biomechanical principles in torque tests are quite complex. Shear force in the bone implant interface is the most important component. The removal torque tests are invasive biomechanical tests that give information about the rigidity of the implant within the bone [42]. The removal torque test shows resistance to shear forces at the bone implant interface, but the results do not always show a direct correlation with bone response or surface hardness.
Therefore, it is also necessary to measure the amount of bone implant contact [43]. On the other hand, it is possible to measure implant stability by resonance frequency analysis in terms of the implant stability quotient (ISQ) at every moment of treatment and loading [44,45]. It is believed that the measurements made by resonance frequency analysis have eliminated the observer dependent errors. Because the transducer is inserted onto the implant and the measurement is automated. It has been reported that the torque used during the insertion of the transducer (Smart Peg) on implant does not affect the test results and that the results obtained with this method are highly reproducible [46]. Studies have shown that bone height supporting implants significantly affects the RFA values [44,47,48]. If the rigidity of the bone implant interface is assessed by RFA, the results are expected to relate to bone density and bone implant contact, as well as changes occurring during healing at the bone implant interface or parallel to the resorption of the peri-implant bone [49].
Although there are many methods to assess implant stability, resonance frequency analysis, a non-invasive method introduced by Meredith et al. [44,50,51], allows monitoring of changes in stability and stiffness at the bone implant interface to allow clinically successful and failed implant differentiation [44]. In addition to the non-invasive use of ISQ values in evaluating implant stability, thismethod has been reported to provide reliable and accurate results regarding the bone-implant interface in early evaluation of implant stability [39, 52]. Meredith et al. [50] have shown that implants with high initial ISQ values have shown that stability can reach a peak plateau with shorter recovery periods and that resonance frequency analysis can be a valuable method for monitoring the connection between the implants and the surrounding bone. Considering these investigations, we decided to combine resonance frequency analysis and removal torque test to measure implant stability in our study.
Primer stability plays an important role in the clinical success of implants. Therefore, the results of resonance frequency analysis performed during surgical application can help physicians to predict the prognosis of the implant. Because of this and because it is possible to observe the amount of change in stability as a numerical value, in our study, resonance frequency analysis was performed twice, one immediately after the implant placement and one on the 28th day. In our study, effects of propolis which is known to have a high antioxidant effect, has been experimentally investigated with local and systemic applications on the implant stability in rabbits. After completing the measurements on day 28, the results of the applied stability tests were evaluated.
Numerous modifications have been made to the implant design or surgical technique to improve implant stability and osseointegration. There are many factors that influence osseointegration. Primer stability, implant surface characteristics, anatomic state of the implant site, prosthesis design, occlusion pattern on healing phase and bone metabolism constitute some of these factors [9]. Recently, researchers have begun to show interest in the idea that implant-related complications may occur due to the free radical-induced tissue damage [9-10]. The results of our work have led us to believe that propolis may have positive effects on implant stability and the application of propolis when primer stability is lower, may be an effective support in achieving the desired level of osseointegration and stability within the same duration. These effects are thought to be more pronounced when propolis is administered systemically.
In order to support osseointegration and increase implant stability, propolis application may have positive effects, especially with systemic administration. However, more studies are needed in this area and our work may form a baseline to find alternative ways of supporting osseointegration, to have a clear understanding of the effects of propolis on implant stability, and to determine the effective dosage and administration pattern to help with bone healing.


