Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Claudia-Elena Pleșca1,2, Carmen Manciuc1,2, Carmen Dorobăț2, Egidia Miftode1,2, Olivia-Simona Dorneanu*1,2, Aida Bădescu1,2 and Luminița Smaranda Iancu1
Received: September 30, 2017; Published: October 13, 2017
Corresponding author: Olivia-Simona Dorneanu, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, Romania; “Sf. Parascheva” Clinical Infectious Diseases Hospital, Iaşi, Romania
DOI: 10.26717/BJSTR.2017.01.000436
Due to important structural changes occurring in the immune system exposed to HIV, the risk of sepsis in HIV-positive patients increases proportionally to the duration of this exposure as well as the presence/evolution of other debilitating pathologies. Streptococcus pneumoniae is one of the main causes of invasive infections in HIV-infected patients, and the risk of severe pneumococcal disease is several times higher than the general population. We present the case of an invasive pneumococcal disease in a HIV-infected patient with a strain resistant to third generation cephalosporines.
Systemic infections pose a continuous threat to HIV-positive patients even with the spectacular development of antiretroviral therapy [1]. The main germs involved in the etiology of infections in HIV-positive immunodepressed patients are encapsulated or with intracellular tropism [1,2]. It has been repeatedly demonstrated that Streptococcus pneumoniae is one of the main causes of invasive infections in HIV-positive patients, and the risk of severe pneumococcal disease is several times higher in this category of population than in the general population [3].
In January 2016, the female patient LM, aged 27 years old, living in a rural area, known as HIV-infected for 22 years , at the third stage of the disease, is admitted in the Infectious Diseases Clinic Iasi, by transfer from a county hospital, for: fever, chills, headaches, nausea, vomiting, and myalgia, symptomatology that started suddenly about 48 hours before the presentation. From the patient’s history results a lack of adherence to antiretroviral therapy, the patient being at the fourth treatment regimen, currently represented by the combination of daronavir/ ritonavir and lamivudine (DRV/RTV + 3TC). The clinical examination makes evidence of a moderatelyinfluenced general status, underweight (BMI = 16.3kg/m²), high fever (39.8ºC), skin pallor, tachycardia, tachypnea, hypotension, somnolence, stiff neck. Paraclinical examinations showed leucopenia (WBC = 3560/mmc), moderate neutropenia (PMN = 750/mmc), anemia (Hb = 9.3g/dL, Ht = 33%), ESR = 120mm/1h, C reactive protein = 148 μg/mL, urea = 65 mg%, creatinine = 1.49 mg%, ALT = 75 UI/L, AST = 120 UI/L, glucose = 125 mg%, CD4 lymphocyte count = 29/mmc, HIV RNA = 1.568.000 copies/ml.
The lumbar puncture revealed an opalescent CSF with 512 nucleated cells/mmc, 92% neutrophils in the differential CSF count, 1.6 g/L albumin, 0.2 g/L glucose, 6.9 g/L chloride. The bacterioscopy of CSF sediment revealed the presence of Gram-positive diplococci. Thoracic radiography did not reveal pathological changes suggestive of a lung infection process, and computed tomography only revealed a moderate degree of diffuse cerebral edema, without the description of an intracranial expansive processes; the abdominal ultrasound examination and transthoracic echocardiography were also normal. After collection of blood cultures and CSF, first-choice therapy with ceftriaxone was initiated, along with pathogenic, symptomatic and antiretroviral therapy. Three days later, CSF cultures and blood cultures indicated the presence of S. pneumoniae.
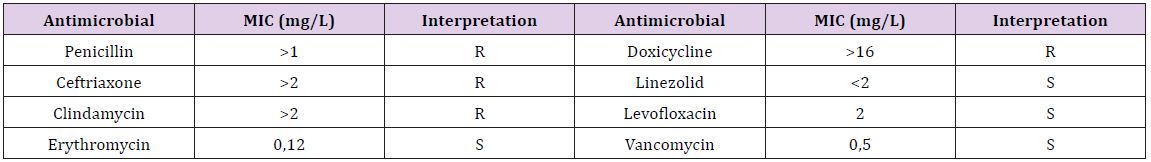
Initial antibiotic therapy was maintained until antibiotic susceptibility test results delivery. The patient’s evolution under treatment was unfavorable, her general condition worsening progressively, with the alteration of consciousness, requiring specific maneuvers of intensive care. At the same time, there was an increase in nitrogen retention syndrome, with increased levels of urea and creatinine (120 mg% and, respectively, 2.2 mg %), hepatic cytolysis and metabolic acidosis (15mEq/L alkaline reserve). The antibiotic susceptibility test indicated resistance ofthe pneumococcal strain to penicillin, ceftriaxone, doxycycline and clindamycin; the isolated strain was susceptible to vancomycin, levofloxacin, linezolid and erythromycin (Table 1). As a result, antibiotic therapy was changed to vancomycin with favorable clinical and paraclinical evolution in about 14 days. The patient was discharged after 21 days of treatment, with the recommendation to continue the antiretroviral therapy at home and to return periodically for viro-immunological reassessment.
Table 1 : Antibiotic susceptibility test and minimum inhibitory concentration (MIC) values.
S. pneumoniae invasive infections are more common among immunosuppressed patients [4]. The incidence of invasive pneumococcal disease (IPD) is approximately 100 times higher among the HIV-infected population than the general population, and recurrences are also common [3,5]. The introduction of antiretroviral therapy has led to substantial changes in the epidemiology of IPD in HIV-positive patients over the last decades [6]. Studies on this subject have shown a decreasing incidence of BPI as an indirect consequence of the implementation of HAART therapy, most likely due to the drug-generated immune reconstruction [5,7]. In USA, even with an effective therapeutic control of HIV infection, the risk of developing IPD was 35 times higher than in seronegative individuals [6].
Other research has led to the conclusion that, on the contrary, the risk remained unchanged in the post-HAART era. However, there is a cumulative risk factor involved in the determinism of severe infections in HIV-infected patients: race, ethnicity, training, drug and/or alcohol use, smoking, co-morbidities, repeated hospitalizations, co-infection with hepatitis viruses, low levels of CD4 T lymphocytes, high viremia, adherence and, last but not least, patient’s compliance with antiretroviral therapy [7].
In this case, we may state that the long-term evolution of HIV infection (over 20 years, under the conditions of an infection in childhood) and non-adherence to HAART therapy (with a direct effect on the functionality of the immune system), has favored the emergence of pneumococcal bacteremia and meningitis, apparently in the absence of respiratory infection, usually associated with such a pathological condition. The initial lack of response to the empirical antibiotherapy, as well as the need for a change according to the antibiogram results, poses various therapeutic problems of severe systemic infections. The susceptibility to antibiotics of S. pneumoniae producing severe systemic infections was significantly diminished in the last years, the antibiogram being essential in guiding the therapy.
The failure of empiric treatment is a clear demonstration for the need of antibiotic susceptibility testing for S. pneumoniae infections. The particularity of the case also concerns aspects related to the favorable evolution of this patient, probably due to infection with a slow progressive HIV strain, the absence of associated comorbidities and the prompt change of antibiotic therapy.


