Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Erick VG Komba*
Received: September 12, 2017; Published: October 05, 2017
Corresponding author: Erick VG Komba, Senior lecturer, Department of Veterinary Medicine and Public Health, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, P.O. Box 3021, Morogoro, Tanzania
DOI: 10.26717/BJSTR.2017.01.000411
Purpose: Campylobacter mediated diarrhoea is a leading cause of gastroenteritis worldwide. The organisms colonize the gastrointestinal tract of different animal species without causing disease symptoms. Humans acquire infections through contact with or consumption of contaminated meat especially raw/undercooked poultry meat. The rapid emergence of antibiotic-resistant Campylobacter strains heightens the public health concern of the organisms. The aim of this review was to summarise information on the epidemiology and antibiogram of Campylobacter in humans and animals in East African countries.
Method: A structured literature search of PUBMED and Science Direct electronic databases.
Results: Forty reports on thermophilic Campylobacter were identified in four of the five East African countries in the following order; Kenya (16), Tanzania (17), Uganda (4) and Rwanda (3). No study was found to report thermophilic Campylobacter infections in either humans or animals in Burundi. Studies on animals reported colonization of both domestic and wild species. Of the studies that described Campylobacter infections in humans, both symptomatic and asymptomatic subjects were reported to be infected; with higher prevalence in subjects younger than five years old. Among isolates, some demonstrated antimicrobial resistance.
Conclusion: Available information for both human and animal Campylobacteriosis in the region is however sparse thus calling for more research to better understand the epidemiology of infections caused by the organism including clonal dependence and independence of human and animal derived isolates. This understanding will help researchers and health program developers in designing and implementing effective control strategies. Since the organism is zoonotic its control strategies should adopt the “One Health” approach involving collaborative efforts from veterinary and human medicine.
Keywords : Adults; Children; Campylobacter; Chickens; Diarrhoea; Food animals; Wild animals
Abbreviations : CEB: Campylobacter Enrichment Broth; CCDA: Charcoal Cefaperazone Deoxycholate Agar; PCR: Polymerase Chain Reaction; MALDI-TOF: Matrix-Assisted Laser Desorption/Ionization Time of Flight
Campylobacter is recognized as a major cause of human acute food-borne bacterial enteritis both in developed and developing countries [1]. The organism exists as normal flora in the intestinal tracts of many domesticated and wild avian and mammalian species [2-4]. The colonization, by these organisms, of animals used in food production (including poultry, cattle, sheep and swine) and rapid emergence of antibiotic-resistant Campylobacter strains is currently of particular public health concern [5]. Animals carrying Campylobacter pose a risk for human infections from contamination of carcasses, milk, and water through wastes and slurries [6-9]. Human illness, frequently associated with Campylobacter jejuni and Campylobacter coli, is characterized by watery to bloody diarrhoea, abdominal pain, fever, malaise, nausea, presence of leukocytes and red blood cells in faeces and/or vomiting [10,11]; and may persist for a week or even longer [12]. Campylobacter infections can also develop to Guillain-Barre´ syndrome (GBS), an autoimmune-mediated neurodegenerative disorder which causes acute neuromuscular paralysis [13,14].
Rational control programs for many infectious diseases require a thorough understanding of their epidemiologies. The present paper provides a review on Campylobacter studies conducted in East African countries, both in humans and animals. Though limited, reviewed information will enhance our understanding of the epidemiology and more importantly antibiotic resistance profiles of both human and animal derived Campylobacter isolates in the region. This will provide a platform to guide the process of devising holistic control strategies for this zoonotic pathogen.
We searched for the papers which described Campylobacter infections in East African countries (Tanzania, Kenya, Uganda, Rwanda and Burundi) in the Pub Med database provided by the United States National Library of Medicine and Science Direct database provided by Elsevier. Reference lists of initially retrieved articles were searched to identify additional studies. In total 38 papers describing Campylobacter infections in East African countries, published from 1983 to the time of the searches, were collected and summarized in this review. All reviewed papers originated from research activities and none from routine diagnosis. During the review of the literature, the data extracted included sample sources and sizes, identification methods, isolation rates and antibiogram of the tested isolates in some of the studies.
Faecal samples represent the specimen of choice for the isolation of Campylobacter species in individuals presenting with gastrointestinal symptoms [15]. In the present review, studies on Campylobacter in humans involved collection of faecal samples from individuals complaining of enteric problems (symptomatic) seeking for medical services in health facilities within the study areas. In some studies involving children in Tanzania [16-18] and Kenya [19,20] faecal samples were also collected from asymptomatic human subjects. In most of the studies the samples were obtained as whole stool specimens but in some few occasions, again for studies involving children, rectal swabs were collected [16,17].
Studies on detection of Campylobacter in animals involved collection of fecal samples from goats, chimpanzees, cattle, ducks, crows, chickens, gorillas, mice, sheep and pigs. From avians the samples were obtained either through collecting cloacal swabs (live birds), collection of droppings in poultry houses or obtaining caecal contents from intestines of slaughtered birds [21-24]. Available reports on investigations of contamination levels of meat products in Tanzania involved collection of swabs from cattle [25] and pig [26] carcasses. In Kenya such investigations involved collection of swabs from dressed chicken and beef meat samples [27].
The viability of Campylobacter spp. can be affected by environmental conditions such as dehydration and oxygen and both freezing and high temperatures [28,29]. During sample collection for detection of these organisms, therefore, transport to the laboratory should be as fast as possible and preferably in suitable transport media, in order to protect the cells from drying out and from the toxic effect of oxygen [28,29]. In the reviewed studies sample collection took into account the fastidious nature of the micro-organism, and so ensured favourable transport conditions. While some studies transported the samples in Cary- Blair’s medium [23,30], a study by Kaur et al. [31] Adopted the use of brucella broth containing 20% glycerol. Transportation of the samples involved the cold chain.
There exist several protocols for detection of Campylobacter species from different samples. Conventional diagnostic procedures, such as culture and microscopy are however routinely used in most medical microbiology laboratories in developing countries for detection of enteric pathogens including Campylobacter [32]. These procedures include enrichment steps, use of selective culture media, biochemical identification, sero-typing, and resistance resting [32]. The methods are useful for characterization of organisms at species and subspecies levels [33]. Molecular methods are known to provide a means for sensitive and rapid detection of enteric pathogens; but their application is limited by their high costs, inhibition caused by faecal constituents [34], and the need for specialized laboratories and equipment.
The detection of Campylobacter organisms in most of the investigations reported by the papers reviewed in this article employed mainly culture methods, particularly the qualitative (enrichment) method and direct plating method. In Tanzania, however, a study conducted in Chimpanzees [31] employed molecular biological method in combination with enrichment culture. The enrichment media employed in most of the cited studies were mainly Bolton broth, Campylobacter enrichment broth (CEB) and Preston broth. The isolation medium employed was mainly charcoal cefaperazone deoxycholate agar (CCDA). For some studies in Kenya, however, Skirrow’s selective medium [35-38]; and antibiotic containing Campylobacter agar [39] and blood agar [30] were used. The use of non selective Trypticase soy agar containing 5% sheep blood [31] and blood agar [18,24]; together with 0.45 μm filters was also reported in some investigations in Tanzania.
A comparative study by Jacob et al. [24] revealed that the method combining filtration and culture on antibiotic-free blood agar (the Cape Town protocol) resulted into significantly higher prevalence than culture on antibiotic containing agar (the Skirrow’s protocol). The superiority of the Cape Town protocol over the Skirrow’s protocol has been documented previously in South Africa [40]. The authors pointed out that the protocol increases both the number of strains and the number of Campylobacter spp. and species of the related genera Arcobacter and Helicobacter isolated from stools.
In the reviewed studies Campylobacter species were mainly identified based on growth temperature preferences, growth in microaerophilic environment, colonial morphology, Gram staining and biochemical characterization of urease, catalase, and oxidase production, as well as sensitivity to nalidixic acid and cephalothin. The studies adopted hippurate hydrolysis test to distinguish between C. jejuni and other species. According to Nakari et al. [41], however, proper phenotypic identification of Campylobacter isolates, especially differentiation between C. jejuni and C. coli based on the hippurate test, might be difficult and could result in false results. It has also been previously reported by Rönner and Lindmark [42] that hippurate hydrolysis test used for species identification between C. jejuni and C. coli is not always reliable.
This may be supported by findings in one of the cited studies in this review [22] in which Polymerase Chain Reaction (PCR) confirmed only 74.1% of 243 isolates identified by hippurate hydrolysis test to be C. jejuni. Some few studies adopted serotyping method [16] and DNA based molecular techniques [18,22,23,31] to identify and type Campylobacter isolates. A study by Komba et al. [18] also adopted a spectrophotometric method, Matrixassisted laser desorption/ionization time of flight (MALDI-TOF) spectrophotometry technique in identification of thermophilic Campylobacter.
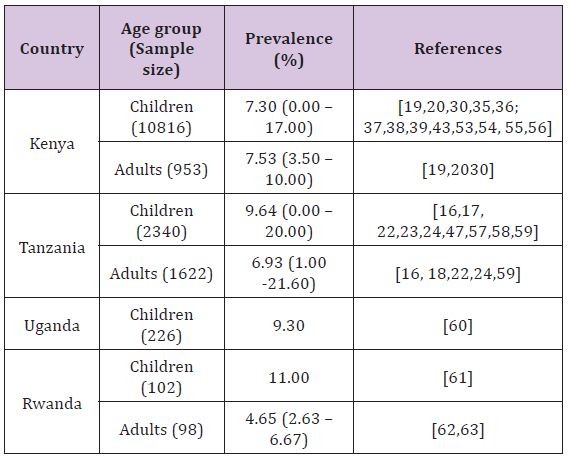
Several studies have reported Campylobacter infections in humans in East African countries with point prevalence ranging from 6.67% (Rwanda) to 9.3% (Uganda) (Table 1). The most common clinical sign in symptomatic subjects was acute gastroenteritis characterized by diarrhoea which in some cases was bloody as reported in Kenya by Brooks et al. [43]. consistently, studies have reported higher prevalence in young individuals, particularly those under the age of 5 years, as compared to adults. It has been mentioned previously that in developing countries campylobacteriosis has been considered as a disease of young children [44,45]; while in industrialized countries it is a disease mainly of adults [46]. This observation can however partly be contributed by the fact that in developing countries children are urgently taken to health facilities even for mild symptoms as opposed to adults. Some studies in Kenya [19] and Tanzania [23] have shown male preponderance in human Campylobacter infections. However a study carried out in Mombasa, Kenya [36], found no significant differences in prevalence between males and females
Studies involving symptomatic and asymptomatic human subjects found no significant differences in prevalence of Campylobacter infections between the two groups; both in Kenya [19,20] and Tanzania [16,47,18]. However in a study by Lindblom et al. [16] analysis of results for a stratum of children under the age of 18 months found a high prevalence of Campylobacter infection in symptomatic subjects as opposed to asymptomatic counterparts. These findings are in line with those obtained by other investigators elsewhere in other developing countries who noticed that Campylobacter is as common in faeces from symptomatic children as from asymptomatic ones but they found a difference among those under the age of 18 months [48-50]. The reason for a high prevalence of Campylobacter infection in symptomatic subjects under 18 months as opposed to asymptomatic counterparts is induced immunity [51].
In most of the studies carried out in the East African countries, C. jejuni was the dominant species isolated and C. coli was less frequently isolated, with the ratio of C. coli to C. jejuni varying considerably being 1.06:1 to 28.41:1 among studies as well as among countries. According to Tadesse et al. [52] Campylobacter species that are most commonly associated with human illness are C. jejuni and C. coli. The author’s further point out that C. jejuni is responsible for up to 90% of the cases of human infections, whereas C. coli is responsible for the majority of the remaining human cases. Their observation supports the findings of the reviewed studies in this article (Table 1).
Table 1: Prevalence of Campylobacter infections in humans in East African countries.
To date thermophilic Campylobacter remain the most common cause of acute bacterial enteritis in humans worldwide [53-63], where the ingestion of contaminated chicken or poor food handling practices associated with raw chicken represents the primary route of transmission [64]. Despite all the acquired knowledge on Campylobacter organisms, including the publication of the complete genome sequence for C. jejuni, the prevalence of human infections remains high and there are still major problems in producing Campylobacter free poultry [65]. The ability of these bacteria to grow at 42oC suggests their adaptation to the gut of most avian species [66]. Of the avian species broiler chickens are commonly regarded as a natural host for this zoonotic pathogen and infected birds carry a very high C. jejuni load in their gastrointestinal tracts, especially the caeca [67]. Colonization of broiler chicks is known to occur at a very early stage in their life and prevalence of colonization among poultry flocks increases gradually with age until slaughter [68] and can reach up to 100% in some areas [69].
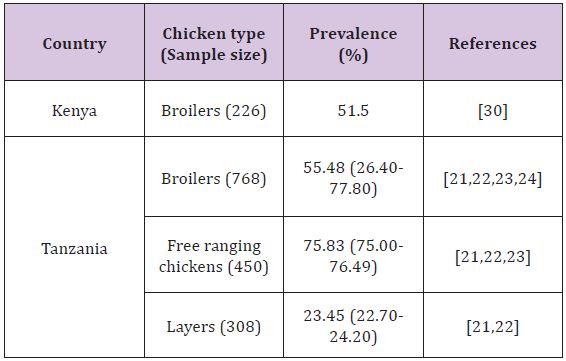
In the East African region, reports on colonization of poultry with Campylobacter are available in Tanzania and Kenya, studies being conducted in chickens as well as ducks. In Tanzania, Nonga and Muhairwa [70] found that 80% of the sampled ducks (n=90) were colonized with Campylobacter organisms; where as in Kenya Turkson et al. [30] isolated the organisms from 29.4% of the screened ducks (n=85). The use of intestinal contents in a study conducted in Tanzania may have attributed to a higher prevalence as opposed to a study in Kenya where cloacal swabs were collected. The prevalence of the organisms in chickens is presented in (Table 2). In all the studies C. jejuni accounted for the majority of Campylobacter detected followed by C. coli. The findings of these studies indicate the role of poultry in the epidemiology of Campylobacteriosis (Table 2).
Table 2: Colonization of chickens by Campylobacter in some East African countries.
Many different animal species maintain Campylobacter species without showing clinical signs. Although the role of these organisms as primary pathogens in farm animals is uncertain, they are of major public health importance [54]. The present review found some studies reporting colonization of farm animals with Campylobacter organisms. A study in Kenya by Turkson et al. [30] found prevalence of 55.1%, 44.0%, 6.3%, 5.8% and 2.0% in diarrhoeic pigs (n=6), healthy pigs (n=150), healthy goats (n=128), healthy cattle (n=121) and healthy sheep (n=98) respectively. In Tanzania goats, pigs, cattle and sheep were found colonized in the order of 4.65% [57,71], 32.2% [26,57,71], 2.25% [57,59] and 31.6% [72] respectively.
In most of the studies in both countries, C. jejuni accounted for the majority of Campylobacter detected in farm animals including pigs. These findings in pigs contradict those of other studies which indicated that Campylobacter infections in pigs show a dominance of C. coli [73-76]. Nevertheless, the finding is supported by some other studies which have found a dominance of C. jejuni in pigs [77,78]. Given these contradictory data, the risk of food borne disease associated with this animal species is not clear but cannot be ignored [79].
. Studies in Tanzania indicated that C. jejuni and C. coli were isolated with similar magnitude in goats. The authors [57,71] found that only goats in households keeping other animals particularly pigs and poultry were positive, carrying isolates similar to those found in these other animals. They concluded that goats are not natural hosts for Campylobacter and those pigs and poultry serve as main sources of infection
In Tanzania Laboratory animals were involved in a study on thermophilic Campylobacter where 30 guinea pigs, 160 mice, 34 rabbits and 242 rats were sampled. Colonization with the organism was detected in 26.7% of the guinea pigs and 1.2% of the rats [72]. In the same country colonization of horses with thermophilic Campylobacter was detected at a tune of 60% (n=5) [72].
Campylobacter species colonize a range of hosts, including domestic animals and wild birds. Campylobacter carrying wild birds have higher chances of contaminating water sources, the environment and food; and eventually transmit the pathogens to humans and poultry [80,81]. In a study conducted in Sweden by Waldenstrom et al. [82], which reported the prevalence of Campylobacter spp. by ecological guilds based on feeding habits; it was reported that migrating birds were commonly infected by Campylobacter spp. A study in Nigeria revealed phenotypical and genotypical similarities among C. jejuni isolates from free flying birds and humans [83]. Several other studies conducted elsewhere in other countries reported occurrence of Campylobacter, particularly C. jejuni, in crows [84-86].
In Tanzania a study by Mdegela et al. [22] isolated thermophilic Campylobacter from crows at 72.8% (n=22). The obtained high prevalence is of epidemiological and public health significance, as it highlights the possibility for crows serving as among important sources of thermophilic Campylobacter in humans and chickens in Tanzania. It has been previously suggested that, free flying birds including crows around poultry farms may transmit thermophilic Campylobacter to chickens if they get access to the rearing houses [80,87]. A study on Campylobacter infections in vervet monkeys in another East African country, Kenya [88] incriminated birds, the main natural carriers of Campylobacter spp [89], as one of the possible sources of infections to the monkeys through environmental contamination with their droppings.
Investigation and understanding of wildlife diseases are vital aspects of natural resource management programs [90]. In the current situation where humans are in frequent interactions with wildlife for different reasons, surveillance and reporting of infectious agents in these animal populations are increasingly important [31]. In this review five studies were conducted in Tanzania (2), Kenya (1) and Uganda (2) to investigate on colonization of wild mammals with Campylobacter organisms. In Uganda studies involving sampling of mountain gorillas (Gorilla beringei beringei) by Nizeyi et al. [91] and Kalema-Zikusoka et al. [92] reported the prevalence of Campylobacter infection at 19% and 8% respectively.
A study on Campylobacter infection in wild mammals in Kenya [88] involved sampling of diarrheic vervet monkeys. Campylobacter jejuni was isolated from all the sampled subjects (n=8). Although captive and free-living wild animals can be healthy asymptomatic carriers of Campylobacter spp., the authors linked infection with the organisms to a fatal outbreak in the study subjects. Taema et al. [90] points out that several other previous research reports showed that Campylobacter spp. were linked to many disease outbreaks in semi-wild and wild animals, with negative effects on the health, productivity, and welfare of a variety of species. In Tanzania, a pilot study by Jiwa et al. [57] isolated C. jejuni from 40% (n=20) of field mice (Mastomis nataliensis). The authors claimed conformity of biotype profiles of the mice isolates with profiles of human isolates obtained in the same study location.
Another study in the same country in which human-habituated chimpanzees (Pan troglodytes schweinfurthii) were the subjects [31], a novel species of Campylobacter, Campylobacter troglodytis, was isolated from the feces of 34% (n=56) of the study subjects. The authors characterized the organism by phenotypic, genotypic, and phylogenetic analyses. The authors however could not determine whether the organism was pathogenic to chimpanzees or not. The ability of this novel Campylobacter to colonize humans and cause enteric disease is an area which needs further investigation.
Poor slaughter methods and unhygienic meat handling may constitute a potential risk of infections to humans [93]. As Campylobacter are among organisms with enteric predilection and stay in the intestinal contents, cross-contamination of meat can originate from the faeces of the same animal or different animals through the slaughterhouse environment or equipment especially during flaying, evisceration or from cross contamination from hide to carcass [94,95]. The use of contaminated water to wash carcasses may also be a source of meat contamination with infectious microorganisms.
Literature search for the present review identified three studies which assessed contamination levels of animal meat products in the East African region. A survey in Tanzania revealed cattle carcass contamination level of 9.3% [25], where as a study in Kenya [27] found beef contamination with Campylobacter organisms at 2% (n=50). Compared with other regions (Asia, Europe and America), many more studies have reported variable levels of cattle carcasses contamination with Campylobacter organisms [95-98]. A study on chicken carcass contamination in Kenya [27] found a point prevalence of Campylobacter organisms on dressed chicken samples of 77% (n=100). The authors reported, C. jejuni, C. coli and C. laridis in chicken carcasses at 59%, 39%, 2% respectively. According to them isolation (85.3%) was higher in chickens that have stayed more than 24 hours since slaughter.
Campylobacter jejuni biotype 1 also featured in a study that isolated the organisms from faecal contents. A survey in Tanzania revealed contamination level in pig carcasses at 10.6% [26]. The level of contamination obtained in this study was comparable to findings obtained elsewhere Aquino et al. (2002). It was however fairly low when compared to studies conducted by Steinhauserova et al. [93] and Malakauskas et al. [99] who reported higher pig carcass contamination ranging from 34-63.6%. In contrast, studies in developed countries; Poland [100], Belgium [101], and Sweden [102] reported low magnitude of carcass contamination.
Differences in Campylobacter isolation from carcasses may be influenced by the prevalence in slaughter animals, abattoir hygiene, slaughter and dressing methods, sampling and analysis methods and sampling plan. Findings from two studies in Tanzania and Kenya incriminated gut content as the major source of carcass contamination. It is suggested from these findings that poultry meat, pork and beef play an important role in the epidemiology of Campylobacteriosis. The findings suggest a need for increased surveillance of Campylobacter in food chains in order to better protect consumers.
Understanding seasonal trends in Campylobacter infections may help in analysis of the seasonal differences in the risks for contracting campylobacteriosis. Evidence of seasonal peaks in human Campylobacter infection has been observed in several European countries [103-107]. Several other investigations have reported seasonality of Campylobacter colonization in poultry with peaks observed in the summer [108-115]. Moreover studies have shown seasonality in the isolation of Campylobacter from retail chicken [116-118]. These findings suggest that climatic factors may be important for Campylobacter infections in broilers and humans in these countries.
In the present review, two studies conducted in Tanzania attempted to investigate the seasonal variation on human Campylobacter infections [16,58], but did not find any significant difference. In Kenya a study by Shimotori et al. [36] found varying human colonization of 17%, 5.4% and 12.2% in July, September and November respectively. However, the authors did not draw any conclusion regarding seasonal variation. The rest of the studies in the region were carried out in different seasons but yet yielded more or less similar prevalence. On the other hand studies on Campylobacter colonization in animals did not strive to investigate the seasonal influence. Similarly, despite being conducted in different seasons, they produced more or less similar results. Previously, however, a study in Zaire (a Central African country) reported high prevalence of Campylobacter infections in humans during the wet season [119].
The use of large amount of antimicrobial agents in modern production animals to control infections tends to select for resistance in the zoonotic bacteria and thereby posing a risk for human health [120-122]. As with several other bacteria, Campylobacter with resistance to antimicrobial agents have been reported in both developed and developing countries. The situation is worse in developing countries where there is widespread and uncontrolled use of antibiotics. Studies indicate an upward trend of Campylobacter resistance to antibiotics with varying patterns being seen in different countries and regions [5,123]. A number of studies in East African countries have addressed the issue of antimicrobial resistance in the organisms. In Tanzania studies on both animals [70,72] and humans [18] report antimicrobial resistance among thermophilic Campylobacter isolates.
Different proportions of resistant isolates have been observed including resistance to fluoroquinolones and macrolides, the drugs of choice for treatment of human Campylobacteriosis. Low levels of resistance have however been reported to be at lower levels for these drugs. In Uganda Mshana et al. [60] reported resistance of human derived Campylobacters to Ampicillin.
In Kenya resistance of human Campylobacter isolates was revealed on metronidazole [30]. In this country an observed sensitivity to erythromycin by 100% of the tested isolates [19] dropped to 48% [43] of the isolates in 23 years period. No reason was however raised as an attribute to this observation. Resistance of various proportions of tested isolates to Tetracycline, Clindamycin, Nalidixic acid [43], kanamycin, Sulphafurazole, Chloramphenical, Cotrimoxazole [19] and Trimethoprim-sulfamethoxazole [20,43] have been reported in the country. Elsewhere C. jejuni has been found to be sensitive to several classes of antibiotics, including macrolides (especially erythromycin), which have been traditionally been utilized as first-line therapy, and quinolones such as ciprofloxacin [5].
However, quinolone resistance in Campylobacter is a rapidly emerging global problem and high levels have been documented in Thailand [124], Spain [125], Hong Kong [126], and India [127]. The widespread use of fluoroquinolones in clinical practice and possible utilization in veterinary practice could be contributing factors for these high levels of ciprofloxacin resistance. Available data on tetracycline show high levels of resistance with an upward increase over time [128-130]. The pattern is probably an attribute of persistent use of tetracyclines in animal husbandry. A described natural horizontal transfer of tetracycline resistance gene (tet(O) gene) without antimicrobial selection pressure between C. jejuni in the digestive tract of chickens [131] may also explain these high rates of tetracycline resistance.
In this paper, we reviewed published papers on epidemiology and antibiogram of Campylobacter in humans and different animal species in East African countries. The available information provides enough evidence of existence of Campylobacter infections both in humans and animals in the region, and highlights their public health implications. Colonization of wild birds and mammals (non human primates inclusive) heightens the concern as some of these are in close proximity with humans. Though limited, investigations on antimicrobial resistance revealed existence of the problem. Controlling infections by the organism requires adoption of ‘One Health’ concept, the worldwide strategy for control of zoonoses.
One of the challenges for researchers in the region countries would be to conduct further work using the more sensitive DNA based techniques leading to validation of clonal independence of the isolates circulating in animals (including the wild) and humans. There is also much to be done in the region in order to understand the pattern and trends of antibiotic resistance in Campylobacter isolates of both human and animal origin. Furthermore, environmental reservoirs of Campylobacter and risk factors for human infections with Campylobacter need to be investigated. The present situation, in which proper countermeasures are lacking, warrants attention to be paid for the sanitary handling of animal products particularly poultry. Risks resulting from proximity with wild animals, more so with non human primates should seriously be addressed. The novel Campylobacter species isolated from the chimpanzees (in Tanzania) warrants further studies especially on its ability to colonize humans and its pathogenicity both in chimpanzees and humans.


