Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nabonita Pal1, Sufia Zaman1, Prosenjit Pramanick1 and Abhijit Mitra2*
Received: August 20, 2017; Published: September 12, 2017
Corresponding author: Abhijit Mitra, Department of Oceanography, Techno India University West Bengal, Salt Lake Campus, Kolkata 700091, India
DOI: 10.26717/BJSTR.2017.01.000348
Heritiera fomes (commonly known as Sundari in India) is gradually getting extinct from high saline pockets of lower Gangetic plain. Hydroponically grown seedlings of the species were analyzed for Chl a, Chl b, total chlorophyll, Chl a:b ratio and carotenoid at five different salinity levels (2, 5, 10, 15 and 20 psu). The concentrations of chlorophyll and carotenoid pigments exhibited significant negative correlations with salinity (p < 0.01). The total chlorophyll expressed, on unit fresh wt. basis decreased by 63.39% to 73.33% and in case of carotenoid the decrease was from 27.78% to 36.84% with the increase of salinity from 2 to 20 psu. The Chl a:b ratio in the plant remained almost constant throughout the period of investigation during 2017 January. The results show that Heritiera fomes of Indian Sundarbans region can be sustained and propagated under low saline environment. At 15 psu, the plants become acclimated in one to two weeks, but at 20 psu the seedlings could not survive. The study is important as rising salinity is experienced in central Indian Sundarbans of lower Gangetic plain due to sea level rise and obstruction of freshwater flow from Ganga-Bhagirathi-Hooghly channel as a result of heavy siltation.
Mangroves are the characteristic littoral plant formation of tropical and subtropical sheltered coastlines [1]. Being on the land sea interface, they are always associated with and subjected to saline seawater. However saline condition is not a prerequisite for their development, rather mangroves choose saline condition to avoid the competition with the more vigorous terrestrial plants. Based on the physiological studies, Bowman [2] and Davis [3] concluded that mangroves are not salt lovers, rather salt tolerants. But excessive saline conditions retard seed germination, impede growth and development of mangroves. Indian Sundarbans, the famous mangrove chunk of the tropics is gradually losing Heritiera fomes (commonly known as Sundari) owing to increase of salinity in the central sector of the delta complex around the Matla River. Reports of alteration of growth in mangroves due to difference in salinity between western and central sectors of Indian Sundarbans are available [4]. However no study has yet been carried on the effect of salinity fluctuation on the photosynthetic pigments and carotenoid level of mangroves under culture conditions from this part of the Indian sub-continent. The effects of salinity on mangroves have been studied in relation to antioxidative enzymes [5,6], leaf structure, rates of transpiration, stomatal conductance and rates of photosynthesis [7,8] and changes in chloroplast structure and function [5,9]. Reported that Na+/H+ antiport catalyzed exchange of Na+ for H+ across the vacuolar membrane of the cells of Bruguiera sexangula offered tolerance to ionic stress imposed by NaCl and this mechanism was important for cellular salinity adjustments. Also, the mechanism of acclimation to salt in mangroves was suggested to be linked to the changes in the vacuolar size in B. sexangula [10]. Further, one of the biochemical mechanisms by which mangroves counter the high osmolarity of salt was accumulation of compatible solutes [5].
In this paper, we present the effect of salinity on pigments in Heritiera fomes under hydroponic culture with an aim to obtain insights into the changes in chlorophyll and carotenoid level with salt acclimation. Such study is important from the point of sea level rise and subsequent saline water intrusion into the islands of Indian Sundarbans as the lower Gangetic delta complex is extremely vulnerable to climate change related effects owing to its location below the mean sea level and experiencing a sea level rise of 3.14 mm/yr. Moreover unlike other mangrove species Heritiera fomes prefer extremely low saline condition and hence can act as biological signal of climate change related to sea level rise.
Seeds of Heritiera fomes were collected from Sundarbans mangrove system of India (Figure 1). Seedlings were raised in the laboratory condition by diluting the water collected from high saline zones of Sundarbans (salinity = 30 psu) under photosynthetically active radiation (PAR) of 1220-1236 μmol m-1 s-1 during January 2017. Two-month-old healthy seedlings were selected for hydroponic culture in Hoagland’s nutrient medium (pH = 5.8-6.0). The preliminary experiments were carried out in the selected species at five different salinities (2, 5, 10, 15 and 20 psu) in order to determine the optimum range of salinities in context to photosynthetic pigments and carotenoids. The cultures were aerated continuously with an air bubbler. The hydroponic cultures were maintained in a culture room under a 14 h photoperiod at PAR of 300 μmol m-2 s-1, 26 ± 30C, and 80% RH. The culture medium was changed every 7 d. Leaves were harvested at 7, 14, 21 and 30 d intervals to measure the pigment concentrations.
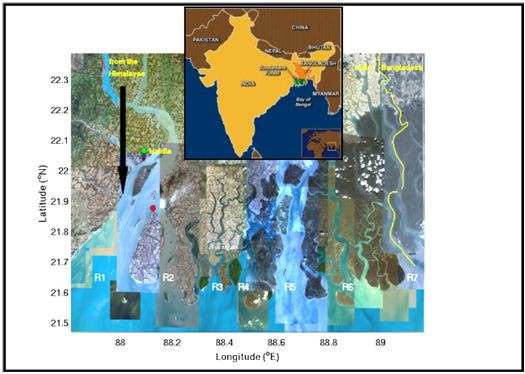
Figure 1: Map of the study region with 2 stations marked in red. The western station is located at 21° 52’ 20.78” N, 88° 7’ 29.73” E, at the tip of Sagar Island. The seven rivers marked by R1 through R7 from west to east are: Hooghly, Muriganga, Saptamukhi, Thakuran, Matla, Gosaba and Harinbhanga. The discharge system of the two metropolises of Haldia and Kolkata (Calcutta) are connected to the two western rivers, which are also fed by the meltwater from the Himalayas after being regulated through barrages. The central station, at 22° 15’ 33.97” N, 88° 39’ 34.64” E, is connected to the rivers R4 and R5 which do not have any freshwater input.
Leaves (0.5 g) were homogenized in chilled N, N-dimethylformamide (DMF) in a mortar and pestle in the dark at 40C and the homogenates were centrifuged at 8800×g for 10 min. The supernatants were collected and absorption spectra at 663.8 and 646.8 nm were recorded using Jasco V-530 UV–vis spectrophotometer for estimation of Chl a, Chl b and total Chl following the procedure of Porra et al. [11]. For the estimation of total carotenoids, leaf tissues (0.5 g) were homogenized in chilled 80% (v/v) acetone; the homogenates were centrifuged at 8800 × g for 10 min at 40C in the dark. The absorbance of the acetone extracts was measured at 663, 645 and 470 nm. Total carotenoids were calculated according to Arnon [12].
Statistical analysis of the results was carried out according to Duncan’s multiple range tests. Data were also subjected to analysis of correlation coefficient (r) in order to evaluate the interrelationship between salinity and selected pigments [13].
We observed that the collected seedlings of Heritiera fomes could tolerate maximum salinity up to 15 psu and could be maintained for more than 30 d. On increasing the salinity to 20 psu the leaves began to fall off after the second week, and thus all the experiments were done up to 30 d in the salinity level 2, 5, 10 and 15 psu treated plants. The unhealthy conditions of the experimental seedlings of Heritiera fomes at 20 psu may be attributed to their ambient salinity in the western sector of deltaic Sundarbans region from where they were collected which usually ranges between 2 psu to 10 psu [14]. Such low salinity in the western part of the study area is due to freshwater discharge from the Ganga-Bhagirathi-Hooghly River that has its origin in the Gangotri Glacier of the Himalayas.
The concentrations of chlorophyll and carotenoid pigments decreased significantly with the increase in salinity (Table 1, Figures 2 & 3). The total chlorophyll decreased by 38.54%, 44.00%, 63.85% and 63.89% at 7, 14, 21 and 30 d intervals respectively due to change of salinity from 2 psu to 15 psu. The Chl a:b ratio in the plant, however, remained almost constant for the species and varied only marginally during the period under observation. In our experiments with differential salinity exposure the Chl a:b ratio yielded a value between 3.00 to 3.41 (Table 1). It appears from the results that high salinity did not affect Chl a:b ratio even though the total chlorophyll content decreased at high salt concentration. A similar trend in carotenoid content, expressed in fresh wt. basis, was observed (Table 1). The pigment decreased by 26.32% at the end of 7 d, 19.04% at the end of 14 d, 33.33% at the end of 21 d and 27.78% at the end of 30 d. The decrease of the selected pigments with aquatic salinity is statistically significant (Table 2). The decrease in chlorophyll content at higher salinity might possibly be due to changes in the lipid protein ratio of pigment–protein complexes or increased chlorophyllase activity [15]. Our results agree with several reports of decrease content of chlorophyll and carotenoids by salinity as reported in a number of glycophytes [16,17]. As the Chl a:b ratio remained unaffected at high saline condition in the selected species, it appears that the light harvesting complex (LHCs) of thylakoid membranes are little altered by salt exposure. Units of all pigments are mg.gm-1 fresh weight; Different letters besides figures indicate statistically different means as at p ≤0.01.
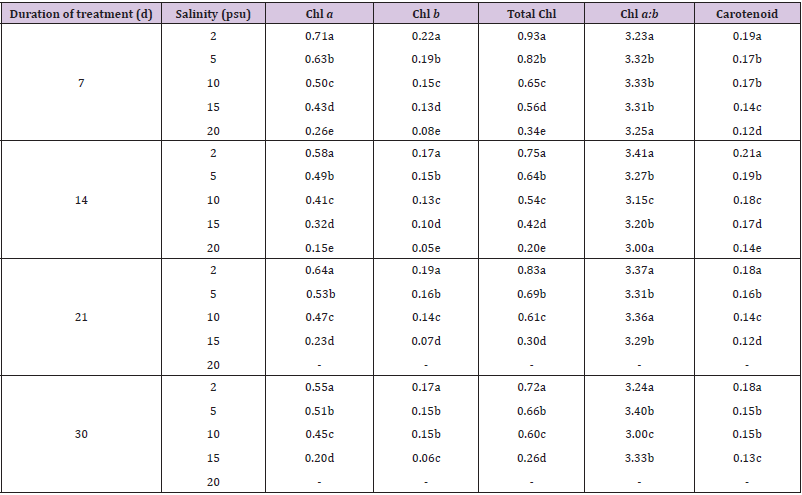
Table 1: Effects of different salinity on pigment concentrations in Heritiera fomes.
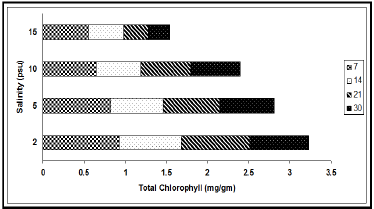
Figure 2: Variation of total chlorophyll content (mg/gm) at different salinities during different exposure days (7, 14, 21 & 30).
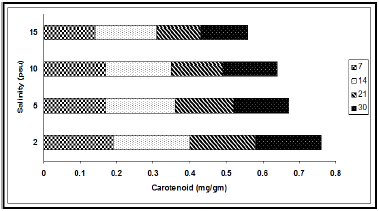
Figure 3: Variation in carotenoid content (mg/gm) at different salinities during different exposure days (7, 14, 21 and 30).
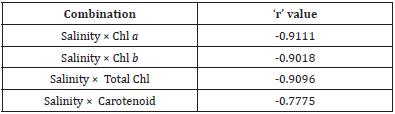
Table 2: Inter-relationships between salinity and selected pigments in Heritiera fomes (all cases p<0.01).
The adverse impact of salinity on leaf chlorophyll of Heritiera fomes may significantly affect the rate of photosynthesis as this pigment is an indispensable raw material for running the process. Till date there have been few studies on the effect of salinity on photosynthetic gas exchange in mangroves. Clough [18] stated in his communication that the rate of light saturated photosynthesis decreases with increasing salinity of ambient media, attributing this to co-limitation of assimilation rate by stomatal conductance and photosynthetic capacity in response to differences in water status induced by the various salinity treatments. Thus, on the evidences available so far it is most likely that salinity exerts its effect on photosynthesis mainly through changes in leaf water status. The present study reveals that the photosynthetic process may be affected at high saline condition due to decrease in Chl a and b concentrations in Heritiera fomes. This present study is different from several previous works as the salinity of water has been altered naturally (through rain water dilution) keeping all the constituent salts of brackish water constant unlike several previous studies where the plants were exposed to different NaCl concentrations [19,20] that are not the real image of ambient seawater. Various studies have shown that a number of mangrove species grow best at salinities between 4 psu and 15 psu [18,21-24] and for Heritiera fomes, the preferred salinity range is much lower [25]. Our results show that Heritiera fomes of Indian Sundarbans region can flourish luxuriantly under low salinity conditions. At 15 psu, the plants become acclimated to salt after one to two weeks of exposure, but at 20 psu the seedlings could hardly adapt.
Indian Sundarbans and its adjacent estuaries at the apex of the Bay of Bengal are one of the less studied regions of the world ocean in context to impact of rising salinity fluctuation on mangrove floral community, although the region sustains the 5th largest mangrove chunk in the world (2120 km2 in the Indian part and 4500 km2 in the Bangladesh part). The present study is extremely important from the point of view of rising salinity in the central sector of Indian Sundarbans over a period of 2 decades [26] due to complete obstruction of the freshwater supply of Ganga-Bhagirathi-Hooghly River as a result of heavy siltation since the late 15th century [25] and rising sea level [27] at the rate of 3.14 mm/yr, which is higher than the global average sea level rise of 2.12 mm/yr and 2.50 mm/ yr along the Indian coastline [28]. The pigments, being the key machinery in regulating the growth and survival of the mangroves require an optimum salinity range between 4 to 15 psu [22,23] for proper functioning.
Heritiera fomes, the freshwater loving mangrove species prefers an optimum salinity between 2 to 5 psu [29]. It appears from our results that the growth of the species would be better if freshwater of the western sector of Indian Sundarbans is channelized to the central sector through capital dredging (initially) and periodic dredging (yearly). The processes will not only help to ecorestore the system through recruitment of freshwater loving mangrove species (like Heritiera fomes, Nypa fruticans, Bruguiera gymnorhiza etc.), but may also help to combat the intrusion of seawater from the southern part of Sundarbans mangrove ecosystem due to expansion of the Bay of Bengal water on account of warming [29,30].


