Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Essam A El-Moselhy1*, Gamal G Elshemy2, Ahmed A Sultan2 and Mohammed A Nafea2
Received: August 09, 2017; Published: September 06, 2017
Corresponding author: El-Moselhy EA, Department of Public Health and Community Medicine, Al-Azhar University, Egypt
DOI: 10.26717/BJSTR.2017.01.000328
Background: Breast cancer in is the commonest and number one cancer among females in Egypt. Female breast cancer (FBC) has many preventable risk factors.
Aim: To determine the socio-demographic, lifestyle, and clinical risk factors of FBC among Egyptian patients.
Patients and Methods: This study was conducted on sample of 400 FBC patients. The patients were recruited from two hospitals of Al- Azhar University in Cairo and Assiut governorates. A case-control study design was used; the patients and controls were subjected for interview for history taking. Odds ratio was used to detect the risk factors.
Results: Significant socio-demographic risk factors were high social, educational, and occupational levels (OR=1.92, 1.69, 1.95; respectively). Significant gynecological risk factors were age at menarche <13, menopause ≥50, and menstrual period ≥40 year (OR=1.49, 1.62, 2.09; respectively). Significant reproductive risk factors were never married, first full term pregnancy at age ≥35, and no breast-feeding (OR=2.7, 2.97, 2.64; respectively). Family history of FBC, the maternal/paternal grandmother and the aunts, sister, and mother were significant risk factors (OR=5.23, 6.68, 5.1, 4.45; respectively). Significant clinical risk factors were history of benign breast disease, heights ≥160 cm, and exposure to environmental factors (OR=5.14, 1.86, 4.94; respectively). Significant lifestyle risk factors were ever smoked, alcohol use, and nonphysically active woman (OR=2.98, 3.51, 1.78; respectively). Significant dietary risk factors were high consumption of fats and low consumption of fresh fruits/vegetables (OR=2.48, 1.73; respectively). Significant health care behavior risk factor was no modification of lifestyle risk factors (OR=2.14).
Conclusions: Many of FBC risk factors are modifiable and preventable; identification of these factors may lead to improve preventive strategies. We recommend to conducted more studies to understand the true epidemiology of FBC. There is need for increase awareness of the disease, strength the national FBC screening program, and integration of the FBC these services into the health facilities that women use.
Keywords: B. cinerea; Chlorophyll fluorescence Myzus persicae; Rate of photosynthesis; Systemic infection
Abbreviations: FBC: Female Breast Cancer; BIM: Body Mass Index; SPSS: Statistical Package For Social Sciences; SD: Standard Deviation; OR: Odds Ratio; CI: Confidence Interval; ECL: Exact Confidence Limits
Female breast cancer (FBC) is the most common cancer in women worldwide [1]. It is affecting one in nine women at some point in their lives in Western communities [2]. The age-standardized rates are 74.1/100,000 in developed countries, 31.3/100,000 in developing, and 48.8/100,000 in Egypt [3]. In the US, FBC is the most commonly diagnosed cancer. It is expected to account for 29.0% of all new cancer diagnoses [4]. Moreover, it represented 31.0% and 15.0% of all cancers and cause of cancer death among women in the US, respectively [5]. Further, it is the second leading causes of cancer deaths in women worldwide [6]. About 45.0% of the annual 300,000 FBC deaths worldwide are occurring in the developing countries [7]. In Egypt, the commonest site of cancer in females is the breast, 38.8%. By 2050, a 3-fold increase in incidence of cancer relative to 2013 was estimated [3]. FBC is influenced by complex interactions between many environmental and genetic risk factors. These interactions might play a major role in its great incidence rates variations in different geographical areas and are still, to somewhat, understood [8].
FBC incidence rates are higher in the developed than developing countries [8]; this could be attributed to the high parity and prolonged periods of breast‐feeding, which are more prevalent in the developing areas and act as protective factors [9]. Further, it is expected that the cumulative incidence of FBC in the developed areas would be lowered by more than one half, from 6.3% to 2.7% by age 70, if females had the mean number of births and lifetime duration of breast-feeding that have been found in the developing areas till recently. Breast-feeding could account for almost 66.0% of the expected lowered in FBC incidence [10]. Trends in lifestyle factors vary greatly between different settings. Some common trends point to considerable increases in FBC due particularly to consistent international trends of younger age at menarche, smaller number of children, late menopausal age, and increasing body mass index (BMI) [11]. The patterns of cancer incidence reflect trends in behaviors associated with its risk, improvements in prevention and control measures, and changes in medical practice. Also, trends in incidence of FBC since late decades (1980s) in the 20th century reflect increases because of changes in female reproductive patterns and the increases detected occult disease during the screening programs [12]. Further, there is a decline in FBC death rate over the past 2 decades. Death rate of FBC is down 36.0% from peak rate as a result of improvements in early detection and treatment [13].
The powers that introduce wider lifestyle changes are linked with urbanization, increased income, altered social and family structures, less physical activities, and more occupations [11,14]. FBC is a characterized by increase prevalence with age increment [15]. Endogenous hormones are the unobvious cause of many risk factors for FBC [16]. Also, positive family history (+ve FH) of FBC is among the most important risk factors. The role of genetic factors in causation of FBC have documented by many clues. Discovering susceptibility genes of FBC supply final support clues [17].
Collectively, FBC risk factors include high social class [18], urban residence [14], early age at menarche, late age at menopause, nulliparity, having the first live birth at a later age, hormonal contraceptive use [19], use of hormone replacement therapy (HRT) [14], history of benign breast diseases [20], other breast cancer [21], and exposure to radiation [11,14]. Lifestyle, modifiable risk factors for FBC are likely to receive changes as smoking, alcohol use [22,23], dietary food intake [24], and obese women with higher BMI [25]; through changes in pattern of diet [24] and physical inactivity [25], all of which are cleared to be carcinogenic [11]. Prevention of FBC must directed to the modifiable risk factors, combat children obesity that leading to early menarche; prevention alcohol use; inducement and facilitate breast-feeding during woman’s work time; maintenance of a normal BMI at post-menopausal ages; and rising levels of physical activity [11].
The aims of the current study are to determine the sociodemographic, gynecologic, reproductive, family history, associated medical conditions, and lifestyle risk factors of FBC among Egyptian patients.
Study design: A retrospective, case-control, hospital-based, analytic study design was chosen to conduct this research.
Administrative design: Approvals to carry out this study in the General Surgery Departments, Al-Azhar University Hospitals at Cairo (Al-Hussein) and Assiut were taken from the Councils of these departments.
Study tools: A specially designed comprehensive interviewing form contains data relevant to topic of the study was used to collect data from the patients and controls. All of them had undergone anthropometric measurements; height (cm) and weight (kg) were measured with participants standing without shoes and heavy garments. Body mass index (BMI) was calculated and classified according to WHO [26].
Ethical considerations: The study was approved by the local Ethics Committee of Al-Azhar Faculty of Medicine. The purposes of the study and procedures that will be to be done were explained to the patients and controls. Consents of both of them were given before recruiting in the study. Confidentiality and security were guaranteed for both of them. The right of the patient to withdraw from the study at any time without any effect on the patient’s right in completing his/her treatment was guaranteed.
The collected data were organized, tabulated and statistically analyzed using statistical package for social sciences (SPSS) version 20 (SPSS, Inc., Chicago, USA). For qualitative data, frequency and percent were calculated; while for quantitative data, mean ± standard deviation (SD) was used. Odds ratio (OR) with 95% confidence interval (CI) or exact confidence limits (ECL) to detect significance of the risk factors was used. Also, t-test was used to detect significance between two means of two groups, the significance level for t-test was accepted if the P-value <0.05.
As respect the features of the studied cases with FBC (Table 1), 39.0% of FBC patients were 50-59, 35.25% ≥60, 17.25% 40-49, and 8.5% were <40 years old. Respecting menstrual status at time of diagnosis, 29.5% and 70.5% of the patients were at pre- and postmenopause, respectively. Regarding history of the same and/or the other breast cancer, 15.5% of the patients had positive history. As regard socio-demographic risk factors of the FBC patients (Table 2), high level of education, secondary and university, was significant risk factor (OR=1.69, 95% CI: 1.46-2.64). Respecting occupation, semi-skilled/skilled and professional occupations represented a significant risk factors (OR=1.69, 95% CI: 1.25-2.29 and OR=1.95, 95% CI: 1.3-2.92; respectively). Collectively, 39.0% of our patients’ belonged to high social class, which represented a significant risk factor (OR=1.92, 95% CI: 1.4-2.63). Whilst, low social class represented a significant protective factor (OR=0.49, 95% CI: 0.36- 0.68). Also, urban residence represented a significant risk factor (OR=1.68, 95% CI: 1.04-2.71).
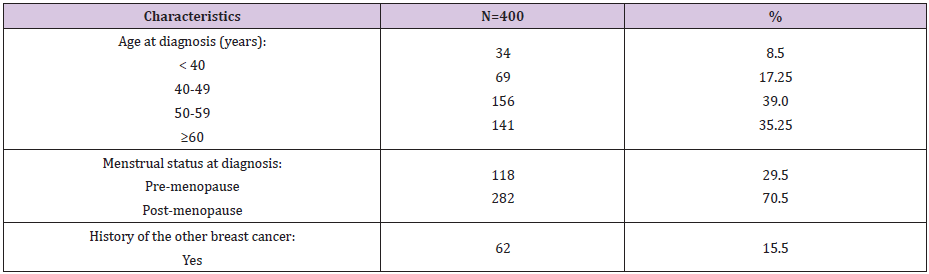
Table 1: Frequency distribution of the characteristic features of the studied female patients with breast cancer.
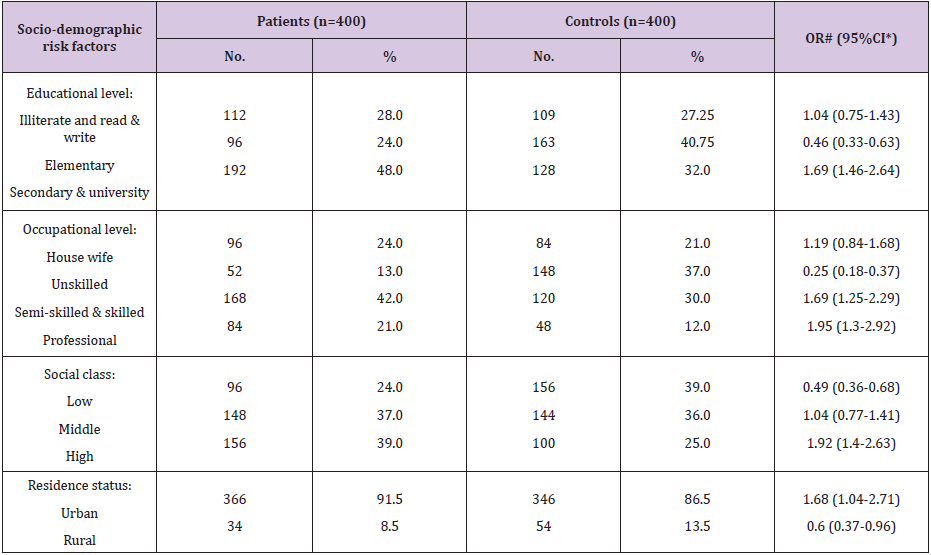
Table 2: Frequency distribution of the studied female patients with breast cancer and controls according to socio-demographic risk factors.
Considering gynecologic risk factors (Table 3), age at menarche <13 year was found among 56.5% of the FBC patients and represented a significant risk factor for FBC (OR=1.49, 95% CI: 1.12- 1.99). Meanwhile, 68.0% of our FBC patients had age at menopause ≥50 year and represented a significant risk factor (OR=1.62, 95% CI: 1.15-2.28). Further, 25.75% of our patients had menstrual period length (time elapsed from menarche to menopause) ≥40 year. This factor represented a significant risk factor for occurrence of FBC (OR=2.09, 95% CI: 1.44-3.04). As regard reproductive risk factors; never married FBC patients were 13.0% and represented a significant risk for FBC (OR=2.7, 95% CI: 1.55-4.73). Also, age at first full term pregnancy ≥35 year was a significant risk factor (OR=2.97, 95% CI: 1.57-5.69). Moreover, nulliparity found to be a significant risk factor (OR=1.83, 95% CI: 1.25-2.69). Never breastfeeding represented a significant risk (OR=2.64, 59% CI: 1.78-3.73). Regarding history of hormonal contraceptive use, it represented a significant risk factor (OR=2.11, 95% CI: 1.52-2.94). As respect pregnancy termination and/or abortion was found among 17.0% of the patients and represented a significant risk factor (OR=2.72, 95% CI: 1.67-4.45).
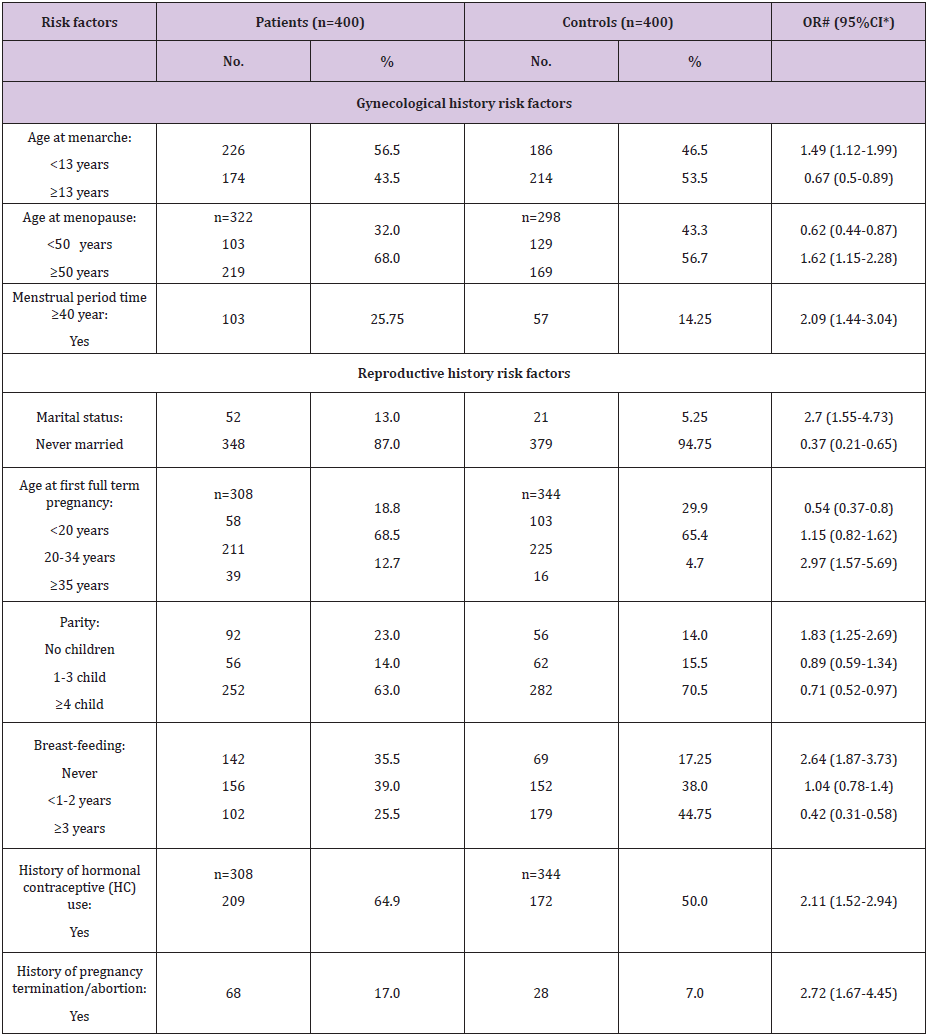
Table 3: Frequency distribution of the studied female patients with breast cancer and controls according to gynecological and reproductive history risk factors.
As regard family history as a risk factor for FBC (Table 4), our results revealed that positive family history (+ve FH) was a significant risk factor (OR=5.23, 95% CI: 2.42-11.7). In details, the significant risk was according to the nearest female relative(s) with FBC; the sister (OR=5.1, 95% ECL: 1.08-48.11), and the mother (OR= 4.45, 95% ECL: 1.21-24.47), the maternal/paternal grandmother and the aunts (OR=6.68, 95% ECL: 1.5-61.29). Regarding age at diagnosis of female relative(s) with breast cancer, it represented insignificant risk. Respecting total number of female relative(s) with breast cancer, the risk increased significantly from 3.83 (95% CI: 1.65-9.21) to 14.47 (95% ECL: 2.18-613.4) for patients with <1 or ≥2 relatives with FBC. Lastly, male relative(s) with breast cancer represented a significant risk factor (OR=9.18, 95% ECL: 1.26- 403.54).
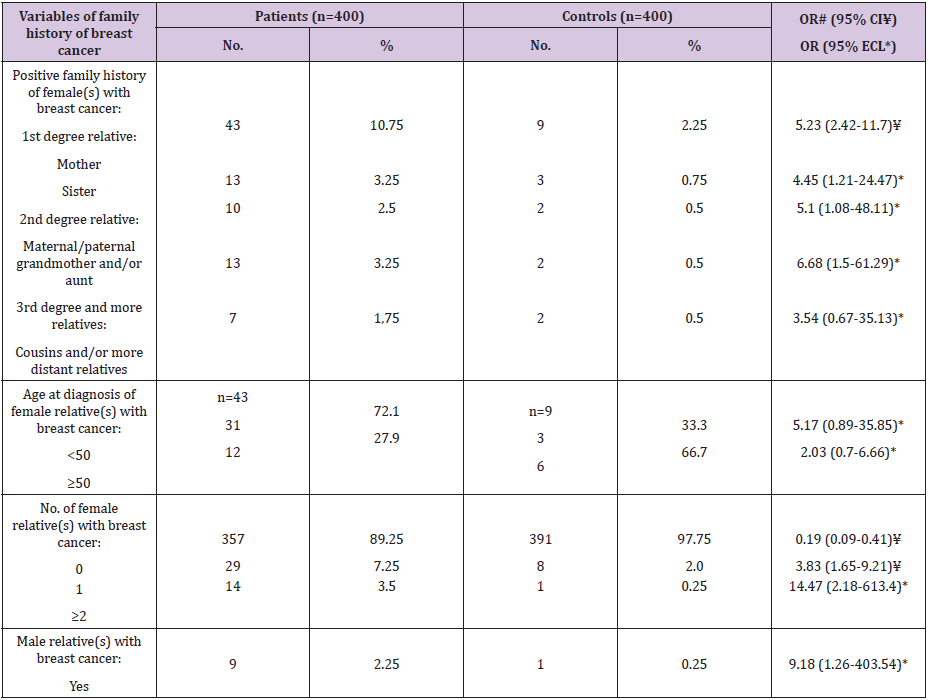
Table 4: Frequency distribution of the studied female patients with breast cancer and controls according to family history of breast cancer.
# Odds ratio ¥ Confidence intervals * Exact confidence limits
As regard FBC associated medical risk factors (Table 5), history of benign breast disease represented a significant risk factor (OR=5.14, 95% CI: 2.94-9.1). Regarding HRT use, it was found to be insignificant risk factor (OR=3.03, 95% ECL: 0.54-30.84). Respecting anthropometric measurements, the height ≥160 cm represented a significant risk factor (OR= 1.86, 95% CI: 1.35-2.56). Also, the mean heights of the patients and controls were 163.37±4.85 and 158.13±3.27 cm, respectively, with a statistically significant difference (P=0.0). Further, mean body weights of the FBC patients and controls were 78.96±6.27 and 73.43±5.37 kg, respectively, with a statistically significant difference (P=0.0). Regarding obesity, BMI ≥30 represented a significant risk factor (OR=1.34, 95% CI: 1.0- 1.78). Respecting history of diabetes mellitus (DM), it was found to be significant risk factor (OR=3.09, 95% CI: 1.98-4.85). Also, exposure to radiation and/or environmental factors was found to be significant risk factors for FBC (OR=4.94, 95% ECL: 1.98-14.74). Also, we noticed that no exposure to sun rays was significant risk factor (OR=1.77, 95% CI: 1.32-2.37).
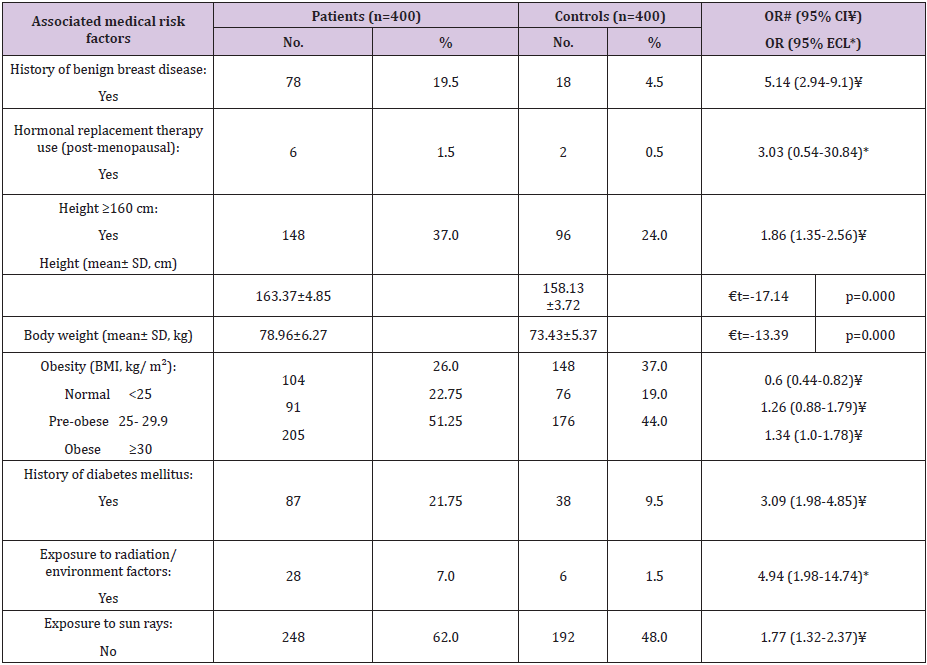
Table 5: Frequency distribution of the studied female patients with breast cancer and controls according to the associated medical and anthropometric risk factors.
# Odds ratio ¥ Confidence intervals * Exact confidence limits € t-test
As regard lifestyle risk factors (Table 6), smoker’s woman represented a significant risk factor for FBC (OR=2.98, 95% CI: 1.83- 4.89). Regarding alcohol use, it was significant risk factor (OR=3.51, 95% ECL: 1.22-12.26). Further, non-physically active women found to be at more risk for FBC (OR=1.78, 95% CI: 1.32-2.4). Lastly, we illustrated that sleep duration <6 hour/day was insignificant risk factor for FBC (OR=1.26, 95% CI: 0.94-1.7). Regarding dietary risk factors (Table 6), high consumption of fat rich foods represented a significant risk factor for FBC (OR=2.48, 95% CI: 1.48-3.33). Further, high consumption of protein and low carbohydrate rich foods represented significant risk factors (OR=1.69, 95% CI: 1.22- 2.32 and OR=1.48, 95% CI: 1.11-1.98; respectively). Lastly, low consumption of fresh fruits and vegetables represented a significant risk for FBC (OR= 1.73, 95% CI: 2.4-7.35).
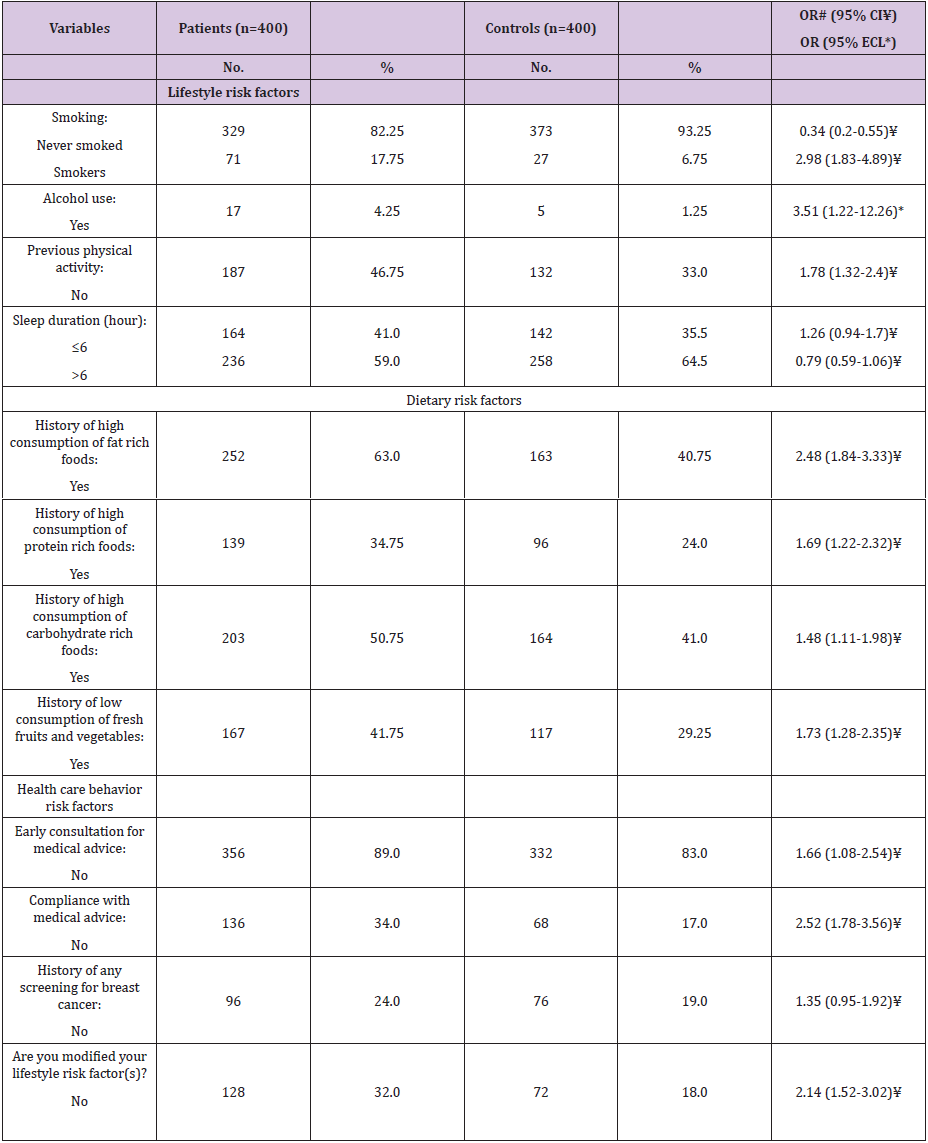
Table 6: Frequency distribution of the studied female patients with breast cancer and controls according to lifestyle, dietary habits,and health care behavior risk factors.
# Odds ratio ¥ Confidence intervals * Exact confidence limits
Respecting health care behaviors risk factors (Table 6), no early consultation and compliance for/with medical advice were significant risk factors (OR=1.66, 95% CI: 1.08-2.54 and OR=2.52, 95% CI: 1.78-3.56; respectively). While, no screening (e.g. mammography) for early detection of FBC was insignificant risk for FBC (OR=1.35, 95% CI: 0.95-1.92). Lastly, no modification of lifestyle risk factors was significant risk factor (OR=2.14, 95% CI: 1.52-3.02).
In the current study, 8.5% of FBC patients were <40 and 35.25% ≥60 year. Darwish et al. [27] found that 10.24% of patients with FBC are <35 year. Meanwhile, Ibrahim et al. [28] reported that 25.4% of their FBC patients are <40 years old. Our figure (8.5%) lies between these figures. Also, CDC [29] reported 3.5%, 28.0%, and 68.5% of FBC cases are in age groups <40, 40-59, and ≥60 years, respectively. As respect menstrual status at time of diagnosis, 70.5% of patients were post-menopause; this result is accordance with Feuer et al. [15]. Regarding history of the same and/or other breast cancer, 15.5% of the patients had positive history; this is consistent with Singletary [20]. In this study, high education level was significant risk factor (OR=1.69). It is cleared that high education level group among the FBC patients had statistically significant difference [30]. On the other hand, it is shown that low education and income levels were linked with high incidence and mortality rates of FBC among whites and blacks in the US [31]. Many of the disparities in cancer incidence linked with race may be caused by factors related with poverty rather than by genetic correlates of race [32]. Lower education level attainment reduces access to medical screening and is often linked with greater exposure to tobacco, alcohol use, poor nutrition, physical inactivity, being overweight and many other risk factors [33].
The present study noticed that upper professional occupations represented significant risk factor (OR=1.95). Collectively, 39.0% of our patients’ belonged to high social class, which represented a significant risk factor (OR=1.92). This result is consistent with Ghadirian et al. [18] and McCredie et al. [30]. The impact of socioeconomic status on occurrence of cancer is almost examined indirectly. But, few studies have separated the impact of poverty and its companion risk factors from genetic differences linked with race [34]. FBC incidence was higher in whites than in other racial and ethnic groups during the last century in the US. Further, its incidence was more among persons with lower income and educational status in high social class. Furthermore, it is more frequently diagnosed at a later stage among persons with low income and educational status, as our patients [31]. Also, this is the case among the African Americans than among whites [5]. The rapid industrialization and consequent socioeconomic transition over the past few decades have been linked by increased FBC incidence rates [35]. The social conditions and ideologies that promoted later marriage, late age at first live birth and women’s entry into the work market, all these factors represent risk factors for FBC. In Egypt, incidence of FBC was also seen more in the high developed areas [14]. Also, urban residence represents a significant risk factor (OR=1.68). It is shown that incidence rate of FBC is approximately 4-fold higher (IR=3.73, 95% CI: 3.3-4.22) in urban than rural areas [14]. Also, this result could be explained; urban residence might equals risky lifestyle, tobacco smoking [18] and rise exposure to environmental hazards. It is believed that plentiful amounts of sunshine could protect from FBC, while working females inside concrete walls and females who live in cloud areas might not received enough vitamin D to get this gift [36].
In the present study, age at menarche <13 years was significant risk factor for FBC (OR=1.49). Endogenous ovarian hormones initiate breast development; regular menstrual cycles induce regular breast cell proliferation until menopause [37]. It is cleared that younger age at menarche increases FBC risk at postmenopausal ages [11]. Also, it is proposed that for a drop of 1.1 years in the mean age at menarche over a 3 decade period; would be linked with a 5.0% increase in incidence rates of FBC [38]. On the other hand, no significant difference is found between FBC patients and controls regarding age at menarche [30]. Meanwhile, age at menopause ≥50 year was significant risk factor for FBC (OR=1.62). McCormack and Boffetta [11] backup our finding. Further, time elapsed from menarche to menopause ≥40 year was significant risk factor for occurrence of FBC (OR=2.09). It is believed that if the patients were under influence of endogenous hormones for ≥ 40 years, it might lead to increase the risk of FBC [16].
As regard never married FBC patients, they were at significant risk to have FBC (OR=2.7). This is accepted and expected, a never married FBC patient means that she hasn’t children, might hasn’t breastfed, and might used fertility drugs; all of them are FBC risk factors. Also, this study showed age at first full term pregnancy ≥35 years was significant risk factor (OR=2.97). This result is consistent with McCormack and Boffetta [11]. Also, more rise in FBC incidence would be occur because of the postponement of childbearing and having less or no children [11], which were part of family planning policies in China [38], Brazil [39], and India [40] between 8.0% and 20.0%, being larger than the increase (<4% in most settings) due to delayed childbearing [11]. Further, we noticed nulliparity was significant risk factor for FBC (OR=1.83). Our finding is supported by McCormack and Boffetta [11]. FBC patients have fewer births than did their controls, 2.2 vs. 2.6 [10,11]. The increase in FBC incidence is due to the reduction in parity of 1-2.5 births in many developing countries worldwide [11].
With lowering parity, life-time duration of breast-feeding will decline. Time trends in per child duration of breast-feeding also vary between countries, they have elevated in most Latin America countries [41] and lowered in many Asian countries [38,40]. Also, we found never breast-feeding represented significant risk (OR=2.64); this was in accordance with McCredie et al. [30]. Further, little parous FBC patients compared with parous controls had ever breastfed (71.0% vs. 79.0%), and their average life-time duration of breast-feeding was smaller (9.8 vs. 15.6 month). The relative risk of FBC decreased significantly by 4.3% (95% CI: 2.9- 5.8) for every 12 months of breastfeeding in addition to a decrease of 7.0% (95% CI: 5.0-9.0) for each birth [10]. Regarding history of hormonal contraceptive use, it was significant risk factor for FBC (OR=2.11). It is believed that the risk is smaller [19]. On the other hand, another study didn’t show that risk [41,42]. As respect pregnancy termination and/or abortion, it was significant risk factor (OR=2.72). Our finding was supported by Ye et al. [43]. On the other hand, another study didn’t found that association [44]. The earlier studies were carried out in societies where induced abortion considered stigma as in our society, while, latter studies were carried out in societies where induced abortion was accepted. The current study cleared that positive family history (+ve FH) was significant risk factor for FBC (OR=5.23); the sister (OR=5.1), mother (OR=4.45), and the maternal/paternal grandmother and the aunts (OR=6.68). Positive FH of FBC in a first-degree relative is a consistent risk factor, risk rises with younger age at diagnosis in the relatives and the number of relatives affected. The relative risk (RR) linked with a maternal diagnosis at age <40 was 2.1 and was 1.5 for maternal diagnosis at age >70 year. For patient with an affected mother and at least one sister affected, RR was increases to be 2.5 [37]. So, FH is a potent risk factor for FBC [45]. Studies observed discrepancy results, further FH prognostic value has not been clearly established [46]. About 20.0% of FBCs are linked with an obvious +ve FH of the disease [47]. However, great studies have demonstrated that <10.0% of FBC might be explained by germ line mutations [48]. Non-BRCA1/BRCA2- linked familial breast cancer can confer a high estimated risk of FBC. Women with 1st degree relative with breast cancer have an estimated 2-fold risk of breast cancer compared with women without FH. So, FH is an established great risk factor for occurrence of FBC [49]. In Egypt, due to the shortage of genetic counseling, FH still represents an important risk factor [17,18]. The lack of this behavior is due to cultural and religious causes among the Egyptian people. It is reported that 25.4% of FBC patients have +ve FH; 13.7% in 1st degree relatives, 10.9% in 2nd or 3rd degree relatives, and 0.8% in far relatives [17].
Our study revealed that the risk of FBC increased significantly (from OR=3.83 to 14.47) with increasing total number of female relative(s) with FBC (with <1 to ≥2 relatives). This was in accordance with our previous study [50]. Also, we showed male relative(s) with breast cancer represented a significant risk factor (OR=9.18). However, the breast cancer risk linked with +ve FH may reflect shared genetic factors, environmental carcinogenic factors among family members or may reflect shared unhealthy lifestyle, dietary and health care behaviors risk factors [50]. Many illnesses and therapies are certainly or doubtfully to induce or to be linked with alterations of hormones and/or growth factors and so might influence FBC risk [37]. This study cleared that history of benign breast disease was risk factor (OR=5.14). This agreed with Armstrong et al. [20]. Also, it is shown that risk of FBC is more in females diagnosed with benign breast disease prior to menopause [51]. Further, it is expected that proportion of cases caused by estrogen and progestin up to 11.0% based on a 30.0% prevalence of use and a 1.4 RR [52]. The present study found that HRT use was insignificant risk factor (OR=3.03). Higher incidence of FBC might be related to more exposure to xeno-estrogens in women used HRT [14].
The current study reported that woman’s height ≥160 cm represented significant risk factor (OR=1.86). Also, we showed the mean heights of the patients and controls were 163.37±4.85 and 158.13±3.27 cm, respectively (P=0.0). It is cleared that for increment of 5 cm of women’s height the RR was 1.02 (95% CI: 0.96-1.10) for pre-menopausal females and 1.07 (95% CI: 1.03- 1.12) for post-menopausal females [53]. Further, mean body weights of the FBC patients and the controls were 78.96±6.27 and 73.43±5.37 kg, respectively (P=0.0). It is shown that increased FBC risk was linked with weight increment and weight is the strongest predictor for FBC (RR=2.85, 95% CI: 1.81-4.49) for women weighing >82.2 kg compared with those weighing <58.7 [54]. The current study observed that BMI ≥30 represented a significant risk factor (OR=1.34). The increased FBC risk is linked with BMI [11,54]. It is shown the inverse relation between BMI and premenopausal occurrence of FBC has been found. Meanwhile, the link between BMI and risk of FBC among post-menopausal women is weak positive [55]. Further, FBC in post-menopausal women would rise up to a 7.5% linked with a 3.2 kg/m2 increase in BMI [56]. Also, our study showed that history of DM is significant risk factor (OR= 3.09). Plasma-based studies introduce great direct clue confirming a link between insulin grades and risk of FBC [57]. Again, we noticed that exposure to radiation and/or environmental factors found to be significant risk factors for FBC (OR=4.94). There is a well-established relation between exposure to ionizing radiation and the risk of developing FBC [58]. Also, higher incidence rates of FBC could be attributed to geno-toxic substances [14]. Further, some findings suggest that organo chlorine exposures, e.g. those found in insecticides, might be linked with a rise in FBC risk [59]. While, other studies did not found that relation between organo chlorines and FBC risk [60].
Also, we noticed that no exposure to sun rays was significant risk factor (OR=1.77). It is cleared women who live in cloudy countries may be at risk, as they not get enough vitamin D to protect themselves against breast cancer. Good amounts of sunshine could be the reason for lower cases of FBC in Mediterranean countries [36]. In many developing areas, the future cancer burden will be worsened by changing lifestyles [11]. Further, changes in cancer incidence rates that happened among international migrants from low- to high-risk countries can be showed, to some extent, as example of the effect of lifestyles changes due to Westernization that could happen on a great scope in developing areas [61]. Migrants from Japan to Hawaii, US had higher rates of FBC than their controls in Japan [56]. This could be explained, changes in patterns of lifestyles of the US society. The present study clarified that smoker’s woman represented significant risk factor (OR=2.98). Active and passive smoking increase FBC risk by 2-fold [18,23]. However, much of the epidemiologic studies viewed the final evidence is controversial and didn’t support cause-effect relationship between passive smoke and FBC [62]. Also, the current work showed that alcohol use is significant risk factor for FBC (OR=3.51). Alcohol use is a documented risk factor for FBC; many epidemiologic studies cleared that increased risk was linked with its use [22,23].
It is believed that women using 35-44 g/day of alcohol compared to non using women had a RR=1.32 (95% CI: 1.19-1.45) [22]. Further, we showed that non-physically active women found to be at more risk for FBC (OR=1.78); women who were adherent to international physical activity recommendations had a significant lower risk for FBC for all pathological types [63]. Reduced levels of physical activity are linked with increased risks for FBC [25]. Vice versa, physical activity may protect against FBC via regulation of gene expression profiles [64]. Further, risk is lowered by about 40.0% among those who were permanently most active [37]. Also, exercise lowers the circulating serum oestradiol level that leads to reduction of the incidence of FBC [65]. Finally, we illustrated that sleep duration <6 hour/day was insignificant risk factor for FBC (OR=1.26). It shown that short sleep duration might be a risk factor for hormone receptor-negative FBC [66]. The present study illustrated that high consumption of fat rich foods represented a significant risk factor for FBC (OR=2.48). It is found that there is a significant difference between FBC patients and controls [67]. Also, dietary fat reduction lowers the circulating serum oestradiol level; this in turn leads to a reduction in the incidence of FBC [65]. Further, we cleared that high consumption of protein and low carbohydrate rich foods represented significant risk factors (OR=1.69 and 1.48, respectively). Again, this result regarding high consumption of protein foods is consistent with Amine et al. [67]. On the other hand, they didn’t found this relation regarding low consumption of carbohydrate rich foods. Finally, low consumption of fresh fruits and vegetables represented a significant risk for FBC (OR=1.73). This result is consistent with Smith-Warner et al. [68]. Recently, it is shown that high vegetable fiber intake could combat the adverse impact of alcohol use [69].
On the other hand, there is low evidence supporting a role for diet components as fiber, fat, coffee or tea, and vitamins (A, C, D, E) in association with FBC risk at pre- and post-menopause [55]. The present study showed that no early consultation forand compliance with medical advice were significant risk factors (OR=1.66 and 2.52, respectively). This is accepted and expected as early detection of benign breast disease might prevent the cancer, while early detection of FBC at early stage might detect it while it’s operable. At the same time, no mammography screening for early detection of FBC was insignificant risk (OR=1.35). Reducing cancer incidence through primary prevention is the most desirable goal [70]. Screening for FBC focuses on detecting occult cancer at an early stage with tumor size preferably <1 cm, with no lymph node and no evidence of distant metastasis to permit early interventions [71]. High coverage rate screening programs had achieved great reduction of FBC mortality [72]. In Egypt, preventive and screening behaviors are not lifestyle practice probably these related to socioeconomic and cultural deeply rooted believes. Finally, no modification of lifestyle risk factors was significant risk factor for FBC (OR=2.14). Again, this is accepted and expected as modifying unhealthy lifestyle (smoking and alcohol use), dietary (low fresh fruits/ vegetables consumption) and health care behaviors (mammography screening) risk factors [50], might prevent the cancer or reduce its morbidity and mortality burden.
Many of FBC risk factors are modifiable and preventable. Identification and appreciation the role of each risk factor for FBC may ultimately lead to improve preventive and therapy strategies; so lower burden of the disease either physically, socially or psychologically. There are many important socio-demographic, gynecological, reproductive, FH, associated medical conditions, and lifestyle risk factors. So, it could be recommended that more studies should be conducted on a big number of patients all over Egypt to understand the true epidemiology of FBC. Also, there is need for increase awareness of the disease, strong national cancer prevention and control strategy, and the integration of the breast cancer screening and preventive services into the health facilities that women use.
We want to acknowledge the patients and controls who participate in this study; also we want to acknowledge the staff members of nurses and administrators who help the authors in finishing this work.


