Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Shikha Rastogi*
Received: July 21, 2017; Published: August 21, 2017
Corresponding author: Shikha Rastogi, Consultant Orthodontist, House number 1224, Sector 9, Faridabad-121006, Haryana
DOI: 10.26717/BJSTR.2017.01.000288
Introduction: The assumption of sterility of packed materials used in orthodontics may lead practitioners to use them without the necessary sterilization. Hence, this study was undertaken to assess the microbial contamination of the orthodontic materials “as received” from the manufacturers and materials exposed to the clinical environment before using it in the patient.
Materials & Methods:Orthodontic materials obtained from 3 different manufacturers (3M, GAC, TP Orthodontics) were divided into 2 categories (“as received” from the manufacturers and “bench top” materials exposed to the clinical environment). Microbiological investigation was carried out using aerobic and anaerobic culture media. Identification of Bacterial species was done and colony forming units (CFU/ml) were measured.
Results: The most commonly used orthodontic materials were found to be contaminated. The species identified were Klebsiella, Streptococci and Citrobacter from elastomeric chains, molar bands, buccal tubes and lingual sheaths.
Conclusion: new packed materials are not always free from bacterial contamination and it is pivotal for manufacturers to state and the practitioners to ensure the sterility of materials before use.
The role of Sterilization is indispensable in daily clinical practice. Although all the instruments used in Orthodontics, as in dentistry, are sterilized before use, the same is not true for orthodontic archwires, brackets, and bands. The orthodontic materials are used “as received” from the manufacturers, often with the assumption that the level of hygiene in the manufacturing process and subsequent transportation is sufficient to allow them for clinical use. In an era, where the orthodontic armamentarium has been upgraded with novel therapeutic systems, it becomes an integral part of the clinical practice to know all relevant aspects of sterilization.
Orthodontic treatment is often performed in the close proximity of gingival tissues. In presence of gingival inflammation, bacterial count is already increased in the oral cavity and if full-banded orthodontic appliances are used then it will further compromise the self-cleansing component of the dentition and oral hygiene practice by the patient. Thus, hyper plastic marginal gingivitis is an almost inevitable result of poor plaque control in patients undergoing orthodontic treatment. In addition, amongst all orthodontic procedures, banding and debanding have a potential threat to gingival tissues. Placement of separators and procedure of banding or even mini-implants may cause trauma to the tissue and a niche for micro organisms. Purmal et al. [1] investigated the sterility of “as received” molar tubes and identified 3 species of bacteria mainly Micrococcus luteus, Staphylococcus haemolyticus, and Acinetobacter calcoaceticus [1]. However, the article does not state the numbers of colony-forming units (CFU) or whether all brackets were contaminated.
Another study investigated the reuse of tungsten carbide debonding burs (classified as reuseable items according to manufacturers) in hospital-based orthodontic departments [2]. Although the purpose of the study was to investigate sterilization methods but it was found that only 24% of departments accurately sterilized them before initial use (Decontamination in Primary Dental Care recommends that all reusable instruments should be sterilized before use) [3]. A survey conducted by J Bagg et al. [4] on Pre-sterilisation cleaning of re-usable instruments in general dental practice revealed that the most common method for cleaning dental instruments was manual washing, with or without the use of an ultrasonic bath.
To date, there have been few investigations of contamination of orthodontic materials “as received” from the manufacturers. We often assume sterility, and yet we have no evidence for this. Therefore, the aim of this study was to evaluate the bacterial load of various orthodontic materials directly received from the manufacturers and also to evaluate the possible contamination, via aerosol or cross-contamination by personnel, of items in the orthodontic environment.
A. To assess the sterility and determine the number of colony forming unit of “as received” and “bench-top exposed” orthodontic materials of 3 different manufacturers.
B. To determine the distribution of bacterial species on “as received” and “bench-top exposed” orthodontic materials of 3 different manufacturers.
There is no microbial contamination of “as received” and benchtop exposed orthodontic materials.
There is microbial contamination present on “as received” and bench-top exposed orthodontic materials.
An in vitro microbiologic investigation was conducted in the Department of Basic Research Science laboratory. The study materials under investigation were divided into two parts i.e. as received materials from manufacturer and bench top exposed materials (Table 1) to the everyday clinical environment (Figure 2). Orthodontic materials were received directly from new stock arrival in the Department of Orthodontics (“as received materials”). The items were exposed to the operatory environment over a period of six months and then subjected to investigation (“clinic exposed materials”). To eliminate any bias, materials from 3 different manufacturers were studied.
Table 1: As received and bench top exposed orthodontic materials included in the study.
Figure 1: Laminar Airflow Chamber with Uv Light.

As received orthodontic materials were directly transported to the lab and the packet is opened under laminar airflow and placed in the sterile tube containing 1ml of reduced transport fluid. Bench top exposed materials were taken from the operatory and placed in the sterile tube containing 1ml of reduced transport fluid and transported to the lab. Too large items mainly archwires were cut into smaller pieces by using sterilized orthodontic instruments and an aseptic technique, with the investigator wearing non latex gloves cleaned with 70%ethanol (Figure 2).
Figure 2: Procedure Followed.
Samples were centrifuged (Figure 3) and plating was done with 100 mL of the sample onto blood agar base in an anaerobic jar for 72 hours. Another blood agar plate was inoculated in a candle jar with 5% CO2 for 48 hrs. 3rd plating was done on Macconkey media and incubated aerobically at 37ºC for 24-48 hours (Figure 4). After the incubation process, identification was done using colony characteristics, gram staining and key biochemical reactions. The growth of bacterial colonies were counted and multiplied with dilution factor of 200 and expressed as colony forming units/ml.
Figure 3: Centrifuge Apparatus.

Figure 4: Centrifuge Apparatus.
The bacterial contamination was notably found in “as received” materials mainly buccal tube, followed by band material and least in e-chain and lingual sheath. The evidence of bacterial contamination was 100% in buccal tubes in all the samples provided by 3 manufacturers. However, the band material sample provided by one of the manufacturer showed 100% contamination. Few samples of E chain and lingual sheath showed contamination. However, the level of contamination was low; the highest being buccal tube at (3.5x 102 CFU/mL). Species identified from the bacterial sample from the items “as received from the manufacturer” are shown in (Table 2).
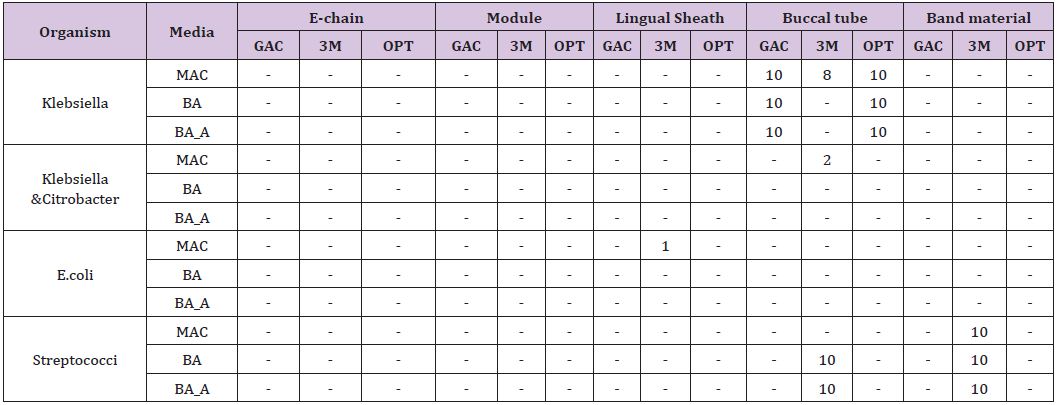
In part 2, “bench top exposed orthodontic materials were cultured similarly to identify the bacterial species and colony forming units/ml. The results are expressed as colony forming units per millilitre of fluid as shown in (Table 3). 100% evidence of contamination was observed in buccal tube samples supplied by 2 manufacturers. However, samples from one of the manufacturer showed 100% evidence of contamination was observed when grown under anaerobic culture media. 5 out of 10 when grown on Macconkey and 8 out of 10 when grown on blood agar showed evidence of contamination. 100% evidence of contamination was observed in band material samples provided by one of the manufacturers, the remaining two manufacturer’s sample showed no growth. 4 out of 10 in the e-chain sample when grown on blood agar media and 3 out of 10 in the lingual sheath sample when grown on Macconkey culture media from one of the manufacturer showed evidence of contamination. However, the level of contamination was low; the highest number obtained was for buccal tube. (5.2x 102 CFU/mL). Species identified from the bacterial sample from the items “bench top exposed from the manufacturer” are shown (Table 3).
Table 2: Materials showing positive bacterial growth.
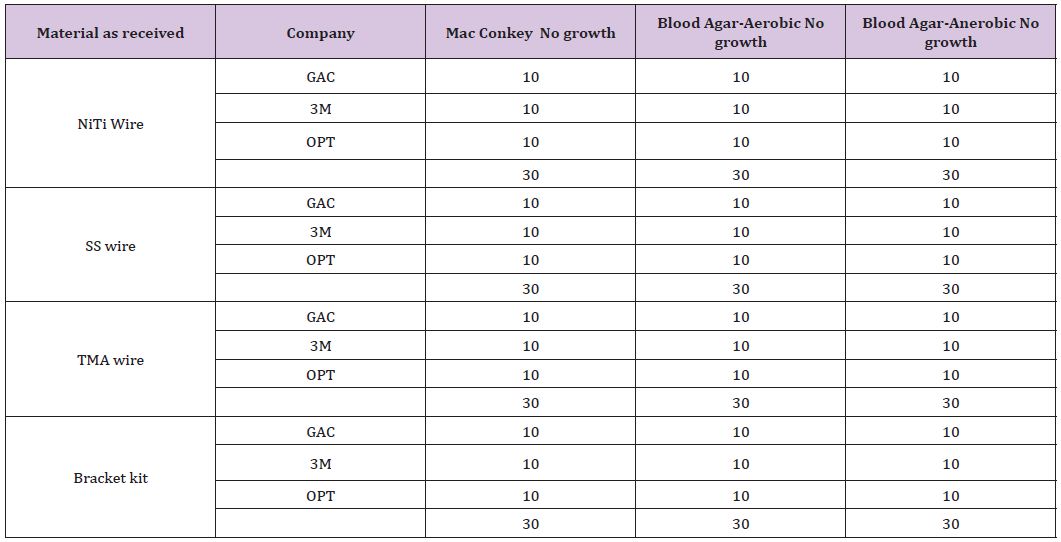
Table 3: Bacterial Species Identified.
The findings of the present research has shown bacterial contamination on “as received” orthodontic materials (Table 3). The level of contamination was found to be low (6000 to 35000CFU/ ml). However, the clinical significance might be low because the oral environment contains 5x108 bacterial cells per unit. The bacterial species isolated in the sample were mainly Streptococcus viridans and Klebsiella pneumonia in buccal tubes and band material. Other species found in low numbers were Citrobacter freundi and E.coli. As opportunistic pathogens, these may cause of a number of nosocomial infections of the respiratory tract, urinary tract, blood and many other normally sterile sites in patients. C. freundii represents about 29% of all opportunistic infections [5]. Virulent strains of E. coli can cause gastroenteritis, urinary tract infections, and neonatal meningitis [6].
In the present investigation buccal tube and molar band material showed maximum level of contamination. These materials have important clinical implications as they come in contact with the gingival tissues and thus the blood stream forming a potential source of cross contamination. Buccal tube and molar band materials are provided in individual sealed packets but no information regarding the sterilization is provided on the packages. The bacterial contamination seen in the as received orthodontic materials could be related to the transmission of bacteria during the manufacturing process, handling or transport.
The amount of bacterial contamination was higher in the bench top exposed materials as compared to the samples studied in part 1. This could be attributed to the aerosol dispersion, improper handling and negligence in the operatory area. Thus it is recommended that the bench top exposed materials should be protected from aerosol dispersion of oral bacteria.
A study designed by Barker et al. [7] investigated orthodontic materials and used tris-EDTA buffer solution to dislodge bacteria from the items under test. This might not have fully dislodged all adherent bacteria which would have underestimated the bacterial contamination. Hauptman et al. [8] investigating dental burs, bathed them in Luria-Bertani broth under aerobic conditions. Once growth was identified, these were further cultured onto Luria-Bertani agar and then sent for bacterial identification by a commercial laboratory using fatty acid profiles and 16S rRNA gene alignment profiles. This method only assessed the aerobic conditions but might have had a greater ability to obtain growth from adherent bacteria.
A questionnaire study by E. M. Roebuck [9] showed that over 90% of the manufacturers did not provide sufficient information for the cleaning of dental instruments and 58% provided insufficient or incorrect instructions for sterilisation. It is clear that the majority of manufacturers of dental devices are in contravention of the Medical Devices Regulations and are thus failing to observe current regulatory requirements [10].
In the present investigation, only the bacterial contamination of orthodontic materials was tested under aerobic as well as anaerobic conditions using 3 different culture media. The method used to dislodge bacteria was reduced transport fluid medium and identification was done on the basis of colony characteristics and key biochemical reactions. Since the level of bacterial contamination was low, it was not further subjected to advance biochemical procedures. On contrary to previous studies [4], Polymerase chain reaction method was not used due to its technique sensitive nature which tends to give false positive results even with low contamination. We have investigated only those as received orthodontic materials which remain in the oral cavity for a long duration of time. A larger sample size would have given a significant result. The sterilization protocols followed during manufacturing and transportation are unknown. Hence, there is a scope for future studies with larger sample size and different materials.
This study highlights the potential areas of contamination that might cause harm to susceptible patients, like immune compromised patients. It would be wise to reduce the possible risks of using potentially contaminated items with additional presterilization procedures before use. Manufactures should clearly state the sterility of their items or advise pre-sterilization before use.


