Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Aranda Cazón C1*, Torrubia Doredo B2, Álvarez López C2, De Gracia Hils Y2 and Cuadrado Cenzual MC2
Received: July 29, 2017; Published: August 21, 2017
Corresponding author:Aranda Cazón C, Pediatrics Service, Hospital Clínico San Carlos, Madrid, España, Spain
DOI: 10.26717/BJSTR.2017.01.000286
Background: The gold standard to assess hyperbilirubinemia in neonates remains the serum bilirubin measurement. Unfortunately, this is invasive, painful, and costly. Trying to overcome these drawbacks, non-invasive methods of bilirubin measurements have been proposed. Our aim was to assess the agreement between capillary sample gas analyzer results, total serum bilirrubin levels and transcutaneous bilirrubin results.
Methods: the transcutaneous bilirubin (TCB) measurements were performed on the infant’s sternum and serum and capillary bilirubin were determined simultaneously. The agreement between both methods was assessed by Spearman´srank correlation coefficient.
Results: A total of 217 measurements were performed in 75infants. Median bilirrubin measurements were 6,127 mg/dl (for serum samples) 6,684 mg/dl (gasometry) and 4,371 mg/dl (TCB). A significant correlation was observed between serum samples and analyzed by gasometry (Spearman´s Rho 0,895; 95% IC CCI: 0.955). Correlation was strong but lower between serum samples and TCB (Spearman´s Rho 0,881; 95% CI CCI: 0.847) and gasometry and transcutaneous samples (Spearman´s Rho 0,914; 95% CI CCI: 0,834). Analyzing for ranges; correlation is higher in 5-9 mg/dl interval comparing serum samples and analyzed by gasometry (95% CI CCI: 0.882).
Conclusion: transcutaneous bilirubin and determined by gasometry could be alternatives to total serum for early diagnosis, proper management of the neonatal jaundice, increase quality of care, improve in parent satisfaction, and an overall decrease in hospital charges.
Neonatal jaundice is a common condition seen in primary care. It is caused by high levels of bilirubin in the blood. Although blood can be sampled routinely from neonates, it could aggressive and painful. Therefore, an accurate and noninvasive method of monitoring of jaundice in such neonates would be desirable. Capillary sample gas analyzer (less accurate sample) and transcutaneous bilirrubin (TCB) determination (fast and painless) have been proposed an alternative to newborns.
Jaundice affects 60% of healthy term neonates during the first week of life. The most common cause of jaundice in newborns is increased unconjugated bilirubin in the blood that is in large part attributable to immaturity of hepatic uptake, conjugation, and secretion of bilirubin. In most of the babies, early jaundice is physiological and harmless, but there are multiple risk factors contribute to severe neonatal jaundice: prematurity, low birth weight, jaundice in the first 24 hours of life, sepsis, lactation failure in exclusive breastfeeding, diabetic mother... High levels of bilirubin can lead to brain damage, which may result in neurodevelopmental impairment such as cerebral palsy, and visual and hearing loss.
There is a global agreement on the early discharge of healthy neonates from hospitals and if neonatal hyperbilirubinemia was not predicted at this stage, the early discharge of these neonates will be leading to an increase in the incidence of kernicterus. Hence early detection of neonatal jaundice is very important, followed by timely referral and appropriate treatment. Indeed, the clinical practice guideline of the American Academy of Pediatrics [1] recommends that all infants who are jaundiced in the first 24 h should have bilirubin measurement, and that all infants should be assessed for risk of significant hyperbilirubinemia before nursery discharge. Recently, use of TCB to screen newborns for hyperbilirubinemia has been suggested as a fast, reasonable alternative to laboratory-based bilirubin testing
The aim of our study was to determine the accuracy and agreement of capillary sample gas analyzer results, total serum bilirrubin levels and transcutaneous bilirrubin results; and examine whether bilirubin measurement and correlation is influenced by clinical factors.
This study was carried out in the intermediate care and in the neonatal intensive care unit of the University Hospital Clinico San Carlos in Madrid, Spain. We determinated total serum bilirubin (TSB), capillary sample gas analyzer (GEM Premier 4000) and transcutaneous bilirubinometer (Dräger JM-103). The range of bilirubin level measured was < 5mg/dl; 5-9 mg/dl; 9-12 mg/dl 12-15 mg/dl and > 15 mg/dl. Significant hyperbilirubinemia was determined by the age-appropriate TSB levels for phototherapy as per AAP guidelines [1]. Clinical decisions about phototherapy were made with the TSB measurement only.
Dräger JM-103 uses two-wavelengths (460 and 550 nm) with a system of dual optical pathway. The device is then placed on the infant’s sternum while lying in the bassinet to determine the TcB level. Each device was configured to display a TcB value derived from the average of three spectral collections when the light source in the device was triggered by the tester. For our study, the transcutaneous bilirubin concentration was measured three times and each measurement lasted approximately 10 to 15 seconds. This was done by the same person and immediately before after capillary blood sampling for serum bilirubin concentration measurements. For analysis, the mean of the three transcutaneous bilirubin readings was taken and then compared with the serum and capillary bilirubin concentration
Clinical data such as gestational age, gender, birth weight, postnatal age, ethnicity, delivery methods (caesarean section vs. vaginal delivery) were extracted from chart reviews. Gestational age (in completed weeks) was based on maternal history of last menstrual period confirmed with ultrasound scan as documented in the hospital records. Exclusion criteria were hemoglobinopathies, hemolysis, evidence of liver disease, hemolytic forms of jaundice, sepsis, suspicious to metabolic disorders and major congenital malformations. We also considered breastfeeding and phototherapy needed. All patient records were anonymized and deidentified prior to analysis. The characteristics of the enrolled infants for each of the two instruments were compared by descriptive analysis. Statistical analysis was performed using SPSS statistical software version 20. Data was analyzed using Spearman´s Rho correlation, IC 95% and Intraclass coefficient correlation (CCI) with variance analysis ANOVA IC 95%.Statistical significance was defined for p < 0.05.
75 newborn infants (44% women and 66% men) were studied. Study population included 41 preterm (54%) and 34 term (46%) newborns (rage 24+1-41+3 weeks). 81.3 % were Caucasian, 12.1 % Hispanic, 4% African and 2.6 % Asian. Median postnatal age was 3.2 days. In total, 46% of neonates were delivered by cesarean while 54 % were vaginal deliveries. 55 % of infants were exclusively breastfed. 217 samples (as some neonates had multiple samples) were collected during the study period (January to May 2015).23 infants received phototherapy, with mean duration of 3.32 days. Median bilirrubin measurements were 6,127 mg/dl (for serum samples) 6,684 mg/dl (gasometry) and 4,371 mg/dl (TCB). A significant correlation was observed between serum samples and analyzed by gasometry (Spearman´s Rho 0,895; CI 95% CCI: 0.955). Correlation was strong but lower between serum samples and TCB (Spearman´s Rho 0,881; 95% CI CCI: 0.847) and gasometry and transcutaneous samples (Spearman´s Rho 0,914; 95% CI CCI: 0,834).
Analyzing for ranges of bilirubin levels; correlation is higher in 5-9 mg/dl interval comparing serum samples and analyzed by gasometry (CCI 95% CI: 0.882). We observed a underestimation of bilirubin concentration (less than 1 mg/dl mg/dl than serum method) with the GEM method at low bilirubin concentrations (<5 mg/dl), whereas at bilirubin concentrations higher than 12 mg/ dl, the GEM method tended to overestimate (about 2-3 mg/dl than serum method).
Comparing serum and transcutaneous bilirrubin, correlation was higher at the 9-12 mg/dl interval (95% CI CCI: 0,882). Globally, TCB understimated serum levels, especially in higher concentrations (>12 mg/dl, lower3-4 mg/dl than serum levels). These results mark the limitations of these devices. Finally, comparing TCB and analyzed by gasometry, we found a strong correlation, higher at medium levels, but TBC also underestimate capillary measurements (similar between serum and TBC). Table 1 and Figures 1-3 summarize the data.
Table 1: Correlation between three methods.
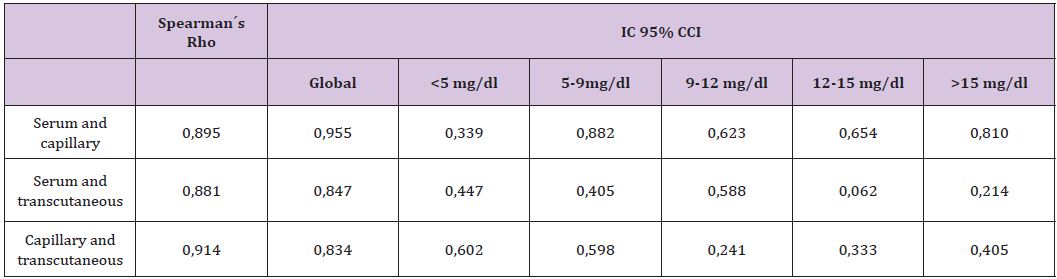
Figure 1: Correlation between serum and capillary bilirrubin.
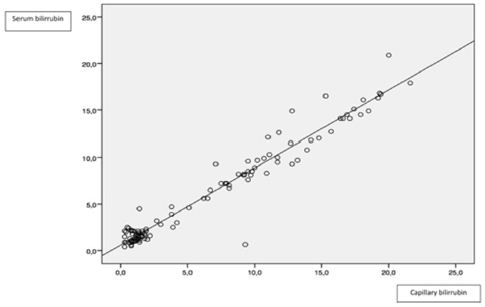
Figure 2: Correlation between serum and transcutaneous bilirrubin.
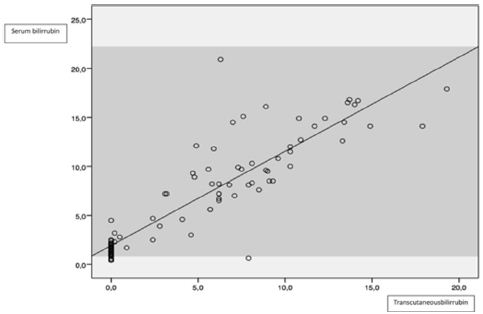
Figure 3: Correlation between capillary and transcutaneous bilirrubin.
The correlations are affected by two clinical variables: the differences between serum determinations and obtained by gasometry were higher when phototherapy was applied (-2.24, 95% CI: -3.05; -1.44) (p <0.001) respect to not applied (-0.11, 95% CI: -0.38; 0.16) (p <0.001); although this determination skims the statistical significant level. This phenomenon is also observed related to gestational age, especially comparing >32 weeks gestation (-1.52, 95% CI: -2.67, -0.37) and <32 weeks gestation (-0.17, CI 95%: -1.17, -0.83). Correlation did not was influenced by clinical factors, except phototherapy need or lower gestational age; these factors associate higher bilirubin levels that could explain it. We found that there were no significant effects of sex, race, delivery methods and birth weight on the difference between three methods.
TSB measurement is the gold standard for detecting and determining hyperbilirubinaemia. Although blood can be sampled routinely from neonates, it is aggressive, painful, might cause local infection, may lead to anemia in the cases of frequent sampling especially in premature newborns and also causes discomfort to infants and anxiety in parents. Methods of measuring the serum levels can be categorized into three groups: “cutaneous” (measuring bilirubin precipitated in the layers of skin), “capillary” (using a spectrophotometer) and “laboratory” (serum bilirubin measurement using chemical reactions). Different studies have evaluated the sensitivity and accuracy of these tools, according to which different results have been obtained.
The GEM Premier 4000® blood gas analyzer provides fast bilirubin results with a small sample volume, faster turnaround time, and can measure other analytes (blood gas, electrolytes, glucose, lactate and co-oximetry). The capillary method, however, is influenced by many variables which, in turn, limit its application as a preliminary option in measuring neonatal bilirubin as well as its potential in becoming a suitable alternative for ordinary laboratory methods. Nonchemical photometric devices give more-accurate information on bilirubin concentrations than do skin test devices, but a blood sample is necessary. The accuracy of these tools on estimating different levels of bilirubin was assessed and it was shown that a significant correlation and similarity existed between bilirubin concentration measured by these tools [2,3]. It is usually been reported that capillary underestimate serum levels; but it has been also reported an overestimation as we founded [4]. Anyway, when the serum level of bilirubin is high, the correlation may be low.
Transcutaneous bilirubin was introduced by Yamanouchi et al. [5] as an easy, safe and convenient method for measuring the severity of jaundice. It is a noninvasive and cost effective method, which as an immediate estimate of total serum bilirubin (TSB) levels and reduces the number of invasive blood samplings. TCB assesses mainly extra vascular bilirubin whereas TSB reflects the intravascular bilirubin concentration and also may be affected by skin pigmentation and skin thickening, making it unsuitable for older neonates [6]. However, in multiple studies, a TcB measurement have been shown to provide reasonably accurate estimates of TSB levels [7-10] and is now becoming a widespread method.
Bilicheck has been well tested in various groups of term or preterm, sick and in multi-racial neonates [11-15]. However, readings above 12 mg/dl should be interpreted with care and should be confirmed with standard laboratory methods, because of the observed underestimation at high concentrations. This method, on its own, cannot be a reliable criterion for exchange transfusion or phototherapy in neonates. It may be useful in identifying infants who do not require phototherapy, but may also identify a high proportion of false positives that is burdensome in resourcelimited settings [16]. Although the JM-103 bilirubinometer tends to underestimate serum bilirubin, especially in patients with high bilirubinlevels, it is a suitable screening tool to identify jaundiced infants that require a serum bilirubin check and may reduce the need for TSB measurement.
As we observed in our study, it has been reported that phototherapy affects the correlation between TCB and blood measurements [17]. In the other hand, it has been reported that the accuracy and reliability of TCB versus TSB is affected by gestational age [18]. In preterm infants transcutaneous bilirubinometry is less accurate than in term infants, as results are affected by the immature skin, the association with higher bilirrubin levels and by a different albumin-to-bilirubin binding [19,20] . Correlation has not be proved to be interfiered by color skin, gender of post conceptional age.
Correlation between gasometry and transcutaneous measurements is strong and useful in medium bilirrubin intervals (5-9 mg/dl). Readings above 12 mg/dl should be confirmed with standard laboratory methods because of the observed underestimation at high concentrations. Transcutaneous bilirubin and determined by gasometry could be alternatives to total serum for early diagnosis, proper management of the neonatal jaundice, increase quality of care, improve in parent satisfaction, and an overall decrease in hospital charges.


