Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Youbi Zakaria ahmed1,2*, Hassan Jouhadi1, Leila Benfaress2, YossiSena3 and Benider Abdelattif1
Received: July 18, 2017; Published: August 01, 2017
Corresponding author: Youbi Zakaria Ahmed, Department of radiation therapy, University Hospital Lyon Sud, Lyon, France 165, chemin du Grand Revoyet 69310, Pierre Bénite, Morocco
DOI: 10.26717/BJSTR.2017.01.000231
Follicular Dendritic cells sarcomais a relatively rare entity with intermediate malignancy. Their evolution is characterized by the frequency of local and/or remote relapses. Due to their rarity, the treatment is not consensual in the literature. We report a rare case of follicular dendritic cells sarcoma of accessory salivary glands in a 45-year-old patient whose progression is favorable after surgery followed by chemo-radiotherapy and review of the literature.
Abbreviations: Follicular Dendritic Cells Sarcoma; Accessories Salivary Glands; Surgery; Chemotherapy; Radiation Therapy
Follicular Dendritic cellstumors are rare entities (less than 1% of malignant salivary gland tumors). Only a few cases have been reported in the literature [1-2]. In addition to their rarity, these tumors shows difficulties in the diagnosis of certainty and in the optimal the rape utic strategy. We report an exceptional case of follicular dendritic cell sarcoma of submaxillary accessory salivary glands in a 45-year-old man and discuss the therapeutic strategy based on data from the literature.
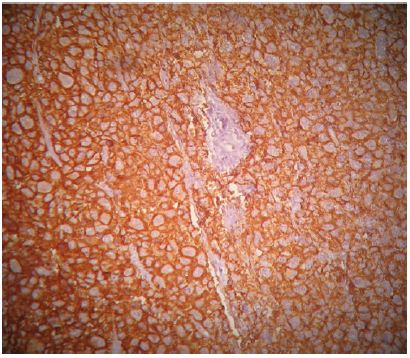
A 45-year-old patient with no previous medical history who consulted for a right latero-cervical swelling, increasing gradually in volume for 3 months, with no pain or other associated otolaryngologic signs. The cervical ultrasound showed a vascularized lesion under the right angulo-mandibular measured 6.5 cm of major axis. The cervical CT scan showed a right sub-angulomandibular tumoallesion develop that the expense of an accessory salivary gland of the inner surface of the lowerlip, mesured 65 x 33mm, with no significant cervical adenopathy or involvement of the parotid glands, Submaxillary or thyroid (Figure 1). The patient had a biopsy excision of the mass. In the macroscopic study, it was an encapsulated mass mesured 7 cm of long axis, of whitish aspect quite homogeneous. Histologically, tumorous proliferation of diffuse architecture was described containing void cells with invisible cytoplasmic boundaries (Figure 2). Exercise margins were healthy at 1 cm. The mitotic index was high (19 mitoses / 10 fields at high magnification), with the presence of some three-pole mitoses. The immuno-histochemical profile was consistent with a follicular dendritic cells sarcoma in full resection, confirmed by the presence of the anti-CD23 antibody (clone MHM6, DAKO, CAPA) But negativity of anti-CD45 antibodies, anticytokératine, and PS100 (Figure 3). Postoperative extension assessement by cervicothoraco- abdomino-pelvic scan was normal.
Figure 1: Cervical tomo densitometry (CT) showing the right sub-angulo-mandibular tumor process developed at the expense of an accessory salivary gland on the inner surface of the lower lip.
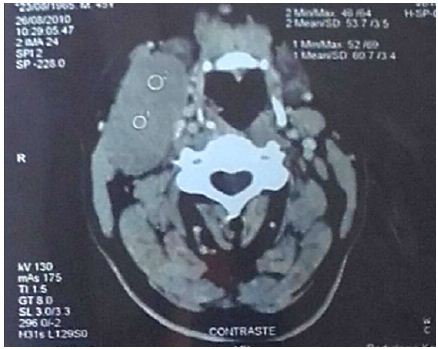
Figure 2: Microscopic aspect of the lesion after staining with hemalun-eosin (at high magnification, x 200) showing tumor proliferation of diffuse architecture containing ovoid cells with invisible cytoplasmic boundaries and round or oval nuclei well nucleolated.
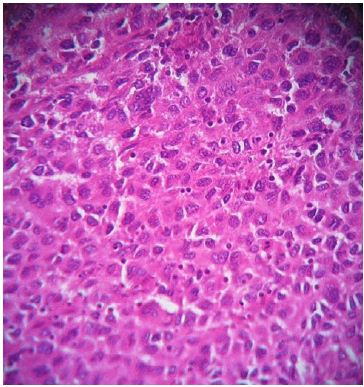
Figure 3: Immuno histochemical appearance after immunolabeling with the anti-CD23 antibody which is positive.
After discussion in a multi disciplinary consultation meeting, the indication of adjuvant chemotherapy and radiotherapy on the tumorbed was posed in front of tumor size, cytologicalatypia and highmitotic index.
The patient received four courses of chemotherapy including Adriblastin, Cyclophosphamide and Cisplatin with good tolerance, followed by conformational radiotherapy on the tumorbed of 50 Gy in 25 fractions of 2 Gy. After a follow-up of 5 years, the patient showed no local or metastaticre currence and therefore considered in complete response maintained.
Follicular dendriti cells (CDFs) are part of the accessory cells of the lymphoid system whose main functionis the presentation of Follicular dendriti cells (CDFs) are part of the accessory cells of the lymphoid system whose main functionis the presentation of the lympho-follicular B antigen. Originally described by Monda et al. In 1986, folliculardendritic cellss arcomare present a rare entity, Belonging, according to the 2008 classification of the World Health Organization (WHO) to the group of tumors with histiocytic and dendritic cells [1]. On the epidemiological level, there appears to be a slight male predominance with a median age of 40 years (9 to 86 years) [2-3]. Castleman’s disease of vascular or plasma hyaline form is found in 10 to 20% of cases [4].
The lymph node involvement is present in 50 to 66% of cases, especially at the cervical level. The most common site of this type of tumoris at the Head and Neck level (mainly palatal tonsils), followed by other localizations (spleen, digestive tract, liver, soft tissue, skin). Macroscopically, follicular dendritic cell sarcoma is generally well-defined, of solid consistency, greyish in color, with in some cases foci of necrosis and haemorrhage [5]. Microscopically, tumor proliferation consists of fusiform, polygonal or ovoid cells of varying architecture (storiform, fascicular, in sheet or nodule, sometimes with windings). The nucleus iselongated, with vesicular chromatin or finely granular and containing a some times prominent nucleolus. The mitotic index is variable from 0 to 10 mitoses / ten fields [2-6]. Our patient had a very high mitotic index at 19 mitoses / 10 fields. On the immuno histochemical (IHC) level, the tumorcells express at least one of the markers of the dendritic follicular cells (CD23, CD35, CD21, CNA42). There was also a positivity for vimentin, EMA in 40-45%% of cases, PS100 in 30-35% of cases and CD68. There is no immuno reactivity for epithelial markers (cytokeratine) and HMB45 [7-8]. In our case, the IHC study showed a positivity of the anti-CD 23.
The main differential diagnosis of follicular dendritic cells sarcoma is the metastases of an undifferentiated carcinoma or an achromic melanoma. However, the absence of expression of cytokeratin AE1-AE3, HMB45 and Melan A by tumor cells makes it possible to eliminate these diagnoses. Another differential diagnosisis interdigital cells sarcoma with clinical and morphological presentation comparable with follicular dendritic cells sarcoma, but it distinguished by the absence of follicular dendritic cells markers expression. The immuno histo chemical study present a major interest for the diagnosis [6-8].
The mixed phenotype of these sarcomas and their incompletely elucidate devolution make the treatment options difficult to define. The sarcomatous phenotype would rather advocate a therapeutic attitude including radical surgery followed by adjuvant radiotherapy whereas the follicular lymphomatous phenotype would rather advocate for isolated radio therapy in stage I or a combination of chemotherapy and local radiotherapy [7,8]. Surgery is the dual, diagnostic and therapeutic reference treatment [3]. There is no specific modality for surgery given the rarity of this type of tumor. Thus, the sarcomatoid phenotype to encourage broad local surgery with sufficient healthy margins. Lymph ademectomy is recommended only in case of macroscopic involved nodes, given the low lympho philicpotential estimate 10% [5]. In the Perkins and Shinohara series, the most important of the literature, there was no evidence to support chemotherapy [3].
It is usually performed in an adjuvant setting or in case of in operable and metastatic tumors. The most commonly used protocolis multi drug therapy, including Cyclophosphamide, Adriamycin, Vincristine and Prednisone [9]. In the same series, 30% of the patients had adjuvant radiotherapy which would seem to be able to reduce the rate of recurrence compared to a single surgery. The doses delivered varied from 59.4 to 70 Gy in conventional fractionation with out precision on the target volumes used, due to the profile of recurrences observed in the literature, especially at a distance. For the parotid localizations The irradiated volume is unilateral including the underlying ganglionic areas in the presence of metastatic adenopathy and in the pure ganglionic case by the addition of sarcoma margins [10]. Our patient had chemotherapy with: Adriblastin, Cyclophosphamide and Cisplatin, followed by conformal radiotherapy of 50 Gy in 25 fractions. The mean five-year survival exceeded 75% for head and neck localizations in the Li et al.serie [4-10]. Finally, the evolution of these tumors is marked by the recurrence in about a quarter of the cases. They are usually late, excluding irradiated volume (in the case of adjuvant radiotherapy) and at a distance from the lungs [8-10].
The head and neck follicular dendritic cells sarcoma constitute a histological entity still in the process of dismemberment. Given the diagnostic difficulties and the mixed phenotypes, an immuno histochemical study must be realized. The therapeutic options must be the subject of an expert multidisciplinary reflection. Carcinological surgery is the reference treatment and adjuvant irradiation of the operative bed should be recommended subject to a level of evidence that remains very low and would require extensive studies. The realization of chemotherapy remains questionable.
All authors contributed to the writing of this manuscript according to the criteria of the ICMJE. All authors have read and approved the final version of the manuscript.
I thank Dr. Sena YOSSI for his kindness and helpful contribution to the drafting of this article.


