Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Prasanta Kumar Mitra1*, TanayaGhosh1 and PrasenjitMitra2
Received: July 14, 2017; Published: July 31, 2017
Corresponding author: Prasanta Kumar Mitra, Professor & Head, Department of Medical Biotechnology, Sikkim Manipal Institute of Medical Sciences, Gangtok, India
DOI: 10.26717/BJSTR.2017.01.000225
Effect of season on in vitro anti oxidant activity of Astilberivularis Buch. – Ham. Ex D. Don leaves was studied. Leaves of Astilberivularis Buch. – Ham. Ex D. Don were collected in different seasons and in vitro anti oxidant activity of the leaves was measured by superoxide anion generation with the help of xanthine-xanthine oxidase assay and with linoleic acid peroxidation assay as well as by DPPH photometric assay. Total phenol, flavonoids, ascorbic acid and carotenoid contents in the leaves of different seasons were also estimated. Results showed that in vitro anti oxidant activity of the leaves of Astilberivularis Buch. – Ham. Ex D. Don was found maximum during rainy season (June – July). Anti oxidant compounds in leaves like total phenol, flavonoids, ascorbic acid and carotenoids were also present in maximum amount during rainy season. Anti oxidant activity, therefore, was related with high content of total phenol, flavonoid, ascorbic acid and carotenoids in the leaves. Leaves of Astilberivularis Buch. – Ham. Ex D. Don of rainy season should be used to get maximum anti oxidant activity.
Keywords: Astilberivularis, Buch. – Ham. Ex D. Don; Anti oxidant activity; Total phenol; Flavonoid; Ascorbic acid; Carotenoids; Effect of season
As early as 1955 Fluck and Pharm showed influence of climate on the active principles in medicinal plants. Thereafter, numerous experiments were conducted in this direction showing influence of season on concentration of active compounds in plants [1-5]. We have also [1] undertaken series of experiments on seasonal variation in amounts of active metabolites in plants and noted that season can change concentration of bio active compounds in different parts of the plants [6-10]. Astilberivularis, Buch. – Ham. Ex D. Don (A. rivularis) is one of the medicinal plants of Sikkim Himalaya. The plant has different names like Buriokahti and Pango in Nepali and in Lepcha respectively [11]. A. rivularis is distributed at a range of 5000 – 9000 feet in Common Temperate of Himalaya and is also found on sloppy waste place. The plant has tall herb stem and leaves are covered with hairs [12].
A.rivularis has many medicinal uses. In traditional medicine juice of the plant is applied to sprains and muscular swelling. Rhizome of this plant is used in curing haemorrhages, diarrhoea, dysentery, headache, prolapse of uterus and to improve fertility [13]. The plant hasanti viral activity [14]. We found anti peptic ulcer activity of A. rivularis leaves in experimental animals [15].A.rivularis is known in literature for its anti oxidant activity too [16]. We also confirmed anti oxidant activity of A.rivularis grown in this region [17]. In this communication effect of season on in vitro anti oxidant activity of A.rivularis leaves is being reported.
A.rivularis leaves were collected from the medicinal plants garden of the University of North Bengal, Dist. Darjeeling, West Bengal, India during Autumn (August – September), Post Autumn (October – November), Winter (December – January), Spring (February – March), Summer (April-May) and in rainy season (June – July) at about 10 am. Leaves were authenticated by the experts of the department of Botany of the said university. A voucher specimen was kept in the department of Medical Biotechnology, Sikkim Manipal Institute of Medical Sciences of the Sikkim Manipal University, Gangtok, Sikkim, India for future references [2].
Collected leaves of A. rivularis were shade dried and powdered. The powder was used as test material.
Chemicals required for the study were purchased from Loba Chem. Lab, Himedia Lab, India and from Merck, Germany
Antioxidant activity of A. rivularis leaves of different seasons was assayed by superoxide anion generation by xanthine-/xanthine oxidase assay [18], linoleic acid peroxidation assay [19] and by DPPH photometric assay [20].
Flavonoids content: Flavonoids content of A. rivularis leaves of different seasons was determined using Aluminum chloride colorimetric method [21].
Total phenols content: Total phenols content of A. rivularis leaves of different seasons was determined by Folin Ciocalteu reagent [22].
Ascorbic acid content: Ascorbic acid content of A. rivularis leaves of different seasons was determined by the method of Cakmak and Marschner [23].
Carotenoids content: Total carotenoids of A. rivularis leaves of different seasons was determined by the method of Jensen [24].
The statistical significance between antioxidant activity values of the extracts was evaluated with a Duncan’s multiple range test (DMRT) at 5 % were considered to be statistically significant [25].
Table 1: Effect of seasons on inhibitory activity of xanthine oxidation, linoleic acid peroxidation and scavenging capacity of DPPH by powdered leaves of A. rivularis.
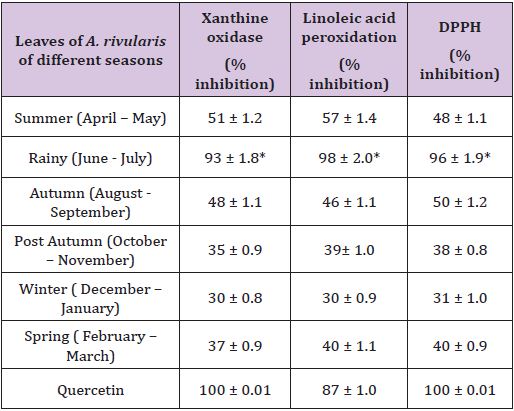
Effect of seasons on in vitro antioxidant activity of powdered leaves of A.rivularis through superoxide anion generation by xanthine-/xanthine oxidase assay, linoleic acid peroxidation assay and by DPPH photometric assay was given in (Table 1). Concentration used: 100 μg / ml. Dose was fixed based on our earlier report [17]. Results were a mean of triplicate experiments ± SD. (Table 1) shows that powdered leaves of A.rivularis of different seasons had more or less in vitro anti oxidant activity but maximum activity was found during rainy season (June – July). Inhibitions in xanthine oxidase, linoleic acid peroxidation and DPPH were found 93%, 98% and 96% respectively. Results were comparable to that of quercetin, a known anti oxidant compound, where inhibition in both xanthine oxidase and DPPH came 100%.
Table 2: Effect of seasons on total phenol, flavonoids, ascorbic acid and carotenoids content of the powdered leaves of A. rivularis.
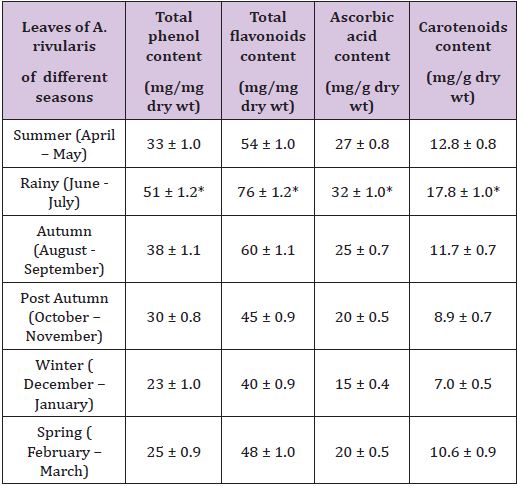
Results were a mean of triplicate experiments ± SD.
Table 2 shows that during rainy season (June – July) total phenol content of the leaves of A.rivularis was 51 ± 1.2 mg/mg dry wt of the leaves. This was maximum when compared to total phenol content of the leaves in other seasons of the year. During August – September (Autumn), October – November (Post Autumn), December – January(Winter), February – March (Spring), April – May (Summer) total phenol content of the leaves were 38 ± 1.1, 30 ± 0.8, 23 ± 1.0, 25 ± 0.9 and 33 ± 1.0 mg/mg dry wt respectively. Same trend was found in total flavonoids, ascorbic acid and in carotenoids content of A.rivularis leaves. All these anti oxidant chemicals were found in maximum amount in leaves during rainy season [5].
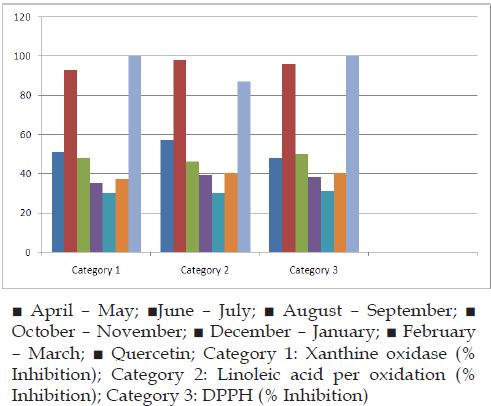
Many plants are known possessing anti oxidant activity [26] and the anti oxidant activity is influenced by season [27]. Recently we have noted in vitro anti oxidant activity of A.rivularis leaves [17]. In present study effect of season on in vitro anti oxidant activity of A.rivularis leaves was studied (Figure 1). Results showed that in vitro anti oxidant activity of A. rivularis leaves in terms of inhibitions in xanthine oxidase, linoleic acid peroxidation and DPPH was maximum during rainy season (June – July) and the anti oxidant activity was comparable to that of synthetic anti oxidant quercetin (Figure 2). Aysel and Sevcan in 2014 showed that antioxidant activity of Prunusamygdalus L. reached the highest value in April for leaves whereas in October for stems [28]. Bahmanzadegan et al. [28] demonstrated the best antioxidant activity of Laurusnobilis was in spring and the lowest one was in winter [29].
Figure 1: Astilberivularis Buch. – Ham. Ex D. Don.

Figure 2: Showing % inhibition of xanthine oxidation, linoleic acid peroxidation and scavenging capacity of DPPH by powdered leaves of A. rivularis in different seasons.
Anti oxidant activity of medicinal plant is mainly due to presence of phenolic compounds, flavonoids, ascorbic acid and carotenoids. These chemicals are responsible for multiple biological effects like free radical scavenging abilities, anti inflammatory and anti carcinogenic activities [30]. We, therefore, studied effect of season on these anti oxidant compounds in A.rivularis leaves. Results (Figure 3) showed that amounts of phenolic compounds, flavonoids, ascorbic acid and carotenoids in the plant leave were maximum during rainy season (June – July). In 2014 Aysel and Sevcan found that highest level of total phenolic compounds in Prunusamygdalus L. was in January for stems while in October for leaves [27]. In vitro anti oxidant activity of A.rivularis leaves during June – July was, therefore, due to accumulation of maximum amount of phenolic compounds, flavonoids ascorbic acid and carotenoids in the plant leaves though commercially available and commonly used in processed food, are not safe. Their toxicity is also matter of concern. It is often claimed that these synthetic anti oxidants have many side effect including carcinogenic activity [30]. Therefore, there are high demands for naturally occurring anti oxidants. As the present study indicates that June – July (Rainy season) is the period when A.rivularis leaves showed maximum in vitro anti oxidant activity, plant leaves of that period may be used as natural anti oxidant.
Effect of season on in vitro anti oxidant activity of A.rivularis leaves was studied. Results showed that in vitro anti oxidant activity of A.rivularis leaves was maximum during rainy season (June – July). The anti oxidant effect was due to presence of maximum amount of phenols, flavonoids, ascorbic acid and carotenoids in the leaves during that period. A.rivularis leaves of rainy season may be used as natural antioxidant.


