Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sankha Bhattacharya1, Bhupendra G Prajapati2* and Arindam A Paul3
Received: July 03, 2017; Published: July 14, 2017
Corresponding author: Bhupendra G Prajapati, Associate Professor, Dept. of Pharmaceutical Technology, Ganpat University, Ganpat Vidyanagar, Mehsana, Gujarat-384012, India
DOI: 10.26717/BJSTR.2017.01.000193
Microbubbles system is a newly invented non-invasive technique in which, in the presence of ultrasound waves or harmonic sounds micropores shrinks and agitated to produce micro bubbles within the applied area. These bubbles have the capability to target any perfused tissues within our body. Recently it was postulated that due to its narrow size range (1 to 10μm vesicular diameter) it can get easily engulf by phagocytic blood cells which can be very effective on lymphocytic cancer. Micro bubbles are prepared by using mechanical agitation, ultrasonication, pressurized gas-liquid mixing system etc. Micro bubbles are made up of a monolayer of protein shells, lipid shells, surfactant shells, polymer shells, Polyelectrolyte multilayer shells. Initially, microbubbles are used as diagnostic tools but due to its versatility of uses and properties of surface conjugation of proteins, genes, micro molecular nutrients, drugs slowly it’s becoming a therapeutic delivery tool. ALBUNEX® (GE Healthcare) was the first albumin conjugated micro bubble which was been approved by US-FDA. Recently importance is been given for brain targeting using Micro bubble carrier system. It was observed that using passive and active transport micro streamed bubbles can able to transport through Blood Brain Barrier (BBB). It is very important to give more importance to its stability and canonization of globules for more industrial acceptability.
Keywords: Ultrasonication; Blood Brain Briers; Microbubbles; Phagocytosis; Brain Drug Delivery System
Abbreviations: BBB: Blood Brain Barrier; PD: Parkinson Disease; CSF: Cerebrospinal Fluid Barrier; PAH: Poly Allylamine Hydrochloride; PSS: Poly Styrene Sulfonate
Due to extensive research and accepting challenges of drug delivery in the brain by scientists, certain brain targeting approaches have been developed during recent times. Some of such approaches like; Osmatic and biochemical BBB dispersion, intra cerebral implants, intra ventricular incisions, BBB carrier mediated and active efflux transport (non-invasive), ligands and chemical peptide bindings, nanoparticular approaches, Trojan house approaches, intranasal drug delivery, using ultrasound webs targeting the brain cells, Avidin-Biotin Technology, genetically engineered molecular antibodies for human therapy, peptide radiopharmaceuticals, nonviral gene therapy has shown some good responses as far as brain drug delivery was concerned. In the recentera, nanoparticular approaches are dominating in research, but it was confirmed that reticule-endothelium system of the brain could not able to uptake nanoparticles during intravenous administration. Nanoparticles did not prove to be a successful approach for brain drug delivery system. Moreover, nanoparticlesovercoated with polysorbit-80 cause indeed some penetration towards the brain, but nanoparticles significance absorption was also been seen in spleen, bone marrow, lungs. Excessive usage of a surfactant such as polysorbit-80 also causes neuronal damage.
Force ultrasound dispersion, a novel approach developed in recent times has its own capability to circumvent BBB and allows drug transport within the brain epithelial tissues. This non-invasive technique has its own advantages over chemically induced nano particle’s, because of its reversible nature. Hynynen has demonstrated that microbubbles intravenous injections with the help of Force Ultrasounds towards brain epithelial were more effective and rapid. Tran cranial application of ultrasound has been used for microbubble delivery of contrast agent as well as for delivery of drug molecules and gene as well.
Paul Ehrlich was the one who identified the Blood Brain Barrier (BBB). He noticed during staining of in vitro circulatory system,almost all the organs were stained except spinal cord and brain. On this context, more eminent scientists like Max Lewandowsky, Edwin Goldman observed limited permeation of potassium ferrocyanate into the brain. On the other hand,goldmen, when injected trypan blue dye within the spinal cord, observed only brain cells gets stained. But all these research has not proven the existence of permissible barrier called BBB. During 1967 Davidson and Spaziani demonstrated that cerebral capillaries are responsible for prevention of diffusion of iodine, sucrose, and p-amino hippurate into the brain. Within the brain capillaries, it was observed that neuron is densely packed and perfused within its microvasculature. Actually, BBB comprising of endothelial cells, tight junctions, central nervous systems blood vesicles, basement membrane, astrocytes, and pericytes [1]. These layers actually acted as a front line defense system for brain and not allows toxic and endotoxins to penetrate inside of the brain. Tight junction assimilated with guanylate, occluding, cingulin, cadherins which are preventing circulating substance to penetrate brain through pure cellular routes. BBB is not allowing larger molecular weight constituents (>500Da), ionic and water-soluble substances to infiltrate inside of the brain. Hence, BBB hinders all macromolecular drugs, diagnostic substances, small molecular agents from penetrating the brain. These obstacles make CNS drug delivery more challenging [2-4].
Drug targeting to the brain is one of the burning questions within the scientific communities as so many CNS disorders and diseases can be wiping out by targeting brain cells. As far as the business point of view is concerned CNS-targeted drug delivery must get some sort of elevation to compete with the cardiovascular market. The major reason for not being given importance by investors in CNS drug targeting is because themajority of drugs are not crossing the BBB to deliver desirable results. Only a few lipid soluble drugs, less molecular weight drugs (<500 Da) can actually cross blood brain barrier. Even though, HIV, brain cancer, Alzheimer disease, amyotrophic lateral sclerosis, Huntington disease, and childhood inborn genetic errors affecting the brain are not effectively can be treated by using conventional lipid soluble and low molecular weight drugs [5,6]. As far as Parkinson disease (PD) were concerned, L-dihydroxyphenylalanine (L-Dopa) treatment was available for several years, but no specific neuroprotective drugs are available to prevent inexorable neurodegradations caused by the PD.
The fluidbarriers are the cumulative cells which inhibit the transport of non-lipid soluble drugs between the environments outside from CNS. The first and foremost is Blood Brain Briers (BBB) which is been associated with the venue, arteriole non fenestrated endothelial of cerebral blood vessels, and complex capillaries. The second one is a cerebrospinal fluid barrier (CSF) comprising with epithelia of the choroid plexus, area post trauma, and median eminence. Followed by blood tumor barrier, epithelial of nasal mucosa, the arachnoid matters-CSF barriers. The lipid or lipid soluble drugs have to cross these potential barriers to delivering atherapeutic effect.
BBB actually acting as a communicative network between CNS and peripheral tissues and regulates the substance exchange between the blood and CNS. Only certain micro molecules, lipids, carbon dioxide, oxygen can cross BBB. Using certain specialized channels ions, glucose, amino acids, and certain nucleic acid components can cross the BBB using suitable channels. Certain hydrophilic molecules such as peptides and amino acids have no specific transport mechanism and have very less absorption as compared to lipophilic molecules, however, the quantity which absorbs inside BBB could capable enough to produce the desired neuroreceptor mediated effect. Certain polypeptides have their own saturable system by which absorption become much easier. BBB is more restricted, but still sometimes some unwanted toxins, therapeutic agent’s crosses blood brain barrier. BBB has also had aneuroimmune function which includes a selection of nitric oxide, prostaglandins, and cytokines. The BBB can be stimulated by one compartment (e.g., the systemic) and simultaneously respond with secretions to the other (e.g., the central nervous system); this function is central to the neurons immune response [7,8].
In cancer therapy or in any diagnostic treatment microbubbles used as diagnostic agents, most recently as a gene and drug carrier microbubbles are been formulating and induced using ultrasounds [9]. Microbubbles have been used in the fermentation of soil, used in hydrophobic plant growth, also used to improve the aquaculture productivity. These have been used to improve the quality of water also used in asewage plant. Bio-medically microbubbles are made up of biodegradable polymers and phospholipids and have similar size of RBC’s used as a diagnostic aid for tissues. Microbubblescarry drugs or genes to any specific tissues and then ultrasound used to burst the microbubbles causing site-specific delivery of bioactive materials. In the absence of ultrasound albumin, conjugated microbubbles can adhere vesicular regions. These microbubbles can able protect glycocalyx damage or endothelial dysfunction. The microbubbleshave some therapeutic property with the addition to that influence of ultrasound makes it more prominent for drug delivery within the cells [10,11]. MicrobubblesComprising of albumin, polymer confining gas like nitrogen, per fluorocarbon has a vesicular diameter of around 1 to 10 μm. These micro-bubbles can able to cross pulmonary capillary and has self-life for few minutes. The ultrasound frequency is maintained around 1-15 MHz. Smaller bubbles can cross capillaries very efficiently and they are a very effective reflector. The intrinsic solubility of the microbubbles approximately 17,000 more than water. In recent advancement ligand, conjugated microbubblesare widely using in the cardiovascular system and tumor diagnosis and therapy [12].
a. Microbubbles should have inject ability.
b. Microbubbles should have ultrasound scattering effect.
c. Micro bubbles are more interactive with vital organs of the body at cellular level; hence they should be bio-compatible.
d. Structurally microbubbles should have an average external diameter between 1-10μm, which is in narrow size to avoid complications when injected into the body.
e. Uniformity of the cell thickness and ligand targeting binding site is very essential for a precise micro bubble.
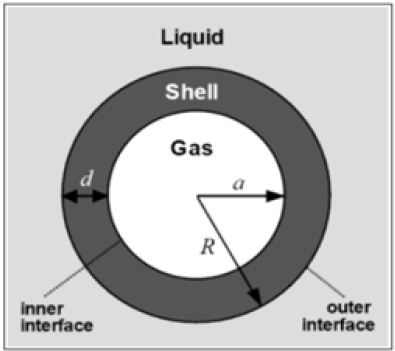
Micro-bubbles comprising of three phases, innermost part is filled with gases, the middle layer or shell layer encloses the gases, outermost layer covered up with the liquid phase (Figure 1).
Figure 1: Components of a micro-bubble.
The albumin shelled microbubbles are very effective in perfusion within the lung capillaries and paved the way for several subsequent formulations that could penetrate left the ventricular system of the heart. ALBUNEX® (GE Healthcare) was the first albumin conjugated microbubble which was been approved by USFDA. These microbubble suspensions was stable for persistent two years, and have 7x108 micro bubbles/mL with a size range from 1 to 15 μm diameter.
Upon sonication of 5% w/v human serum albumin in the presence of air, micro bubbles forms, and which is been encapsulated within 15 nm thick shells of aggregated albumin. For better encapsulation, it is necessary to denaturant albumin using heat prior to sonication. During cavitation, the albumin shells are held together using disulfide bonds, which form cysteine. The relative rigidity was been observed due to ultra-sonication. In ALBUNEX® formulation per fluorocarbon core gas called Optison™ (GE Healthcare) was developed. The low solubility of per fluorocarbon gas gave much longer circulation persistence in vitro. The option™ is currently approved by the US FDA for contrast echocardiography. Not only albumin but ddisulfide bridging occurs between thiol groups found in cysteine amino acid residues, which are present on most proteins can also be produced micro bubbles [13-16].
For proper film stability of microbubbles, it is necessary to maintain the proper ratio of SPAN and TWEEN (approximately 1:1) using Langmuir trough. Surfactants derived from sonicatedmicrobubbles are more stable and capable of reaching higher Langmuir trough. Recently Dressaireet.al had claimed to prepare microbubbles using mono and diesters after blending process at 70 to 75oC wt% glucose syrup. These microbubbles were claimed to have astonishing polygonal domains on their surface [17-19].
Lipid-coatedmicrobubbles are more interesting and inspired by nature otherwise called as bio-inspired micro bubbles. Lipid shells made up of acyl lipids and glycol proteins have good physical stability. USFDA had recently approved the usage of Definite (Lantheus Medical Imaging) and Sonovue® (Bracco Diagnostics) as lipid shell micro bubble for diagnosis purpose. Phospholipids spontaneously assembled into a highly organized monolayer in the presence of an air-water interface. Phospholipids’ aryl chain faces the air and hydrophilic head group faces the water. Thus newly formed microbubbles would circle with self-arranged phospholipidmonolayers. Saturated diacyl phospholipids help to reduce the interfacial tension by which membrane transfer from gellikesafe to liquid crystalline or fluid-like state. Fundamentally lower the surface tension more would be the stability of microbubbles. Moreover, lipid monolayeris more cohesive and has Vender Waals interactions between the highly packed acyl chain makes micro bubbles more ridged. These activities are more important as far as the stability is concerned because superoxide formation from disulphatebinding is actually not that much ridged in the case of a protein-mediated micro bubble. Additionally, lipid membrane is attached with a very weak physical force without any chain entanglement which makes it more effective during ultrasound sonification process. Thus, lipid-coatedmicrobubbleshave exhibited favorable ultrasound characteristics. Finally, lipidcoatedmicrobubble may be functionalized for drug delivery, immune conjugation, molecular imaging etc. Within lipid microbubbles,phosphatidylcholine and lipopolymerare very useful for improving microbubblestability [20-30].
Using cross-linked or entangled polymeric spaces, polymeric microbubbles are organized. Compare to its lipid counterpart, polymeric shells has more resistance area while in the expansion, which makes it a preferablemicrobubble system. In 1990 Wheatley et al developed alginate microbubbles comprising of ionotropic gelation technique. In the reserve were microbubbles were formed by spraying concentrated jets of air within alginate solution. Within the gas-liquid interface infusion of calcium, solution makes micro bubbles more ridge. Microbubbles size usually determined by the flow rate of air around the syringe needle. Microbubbles diameters ranged between 30 to 40μm, which is too large for intravenous administration. During 1997, Bjerkneset a develop a method of making microbubbles with diameters of 1-20μm using double ester polymer with ethylene unit. In the presence of suitable emulsifier and solvent evaporation method, this type of microbubbleswas developed. Optical microscopy and cryogenic electron transmission microscopy had shown that microbubbles had elongated crumpled shapes. The thickness of the polymer shells would be around 150 to 200nm. In another study in 1999, Nayarit and Wealth categorically reported poly (D, L-lactide-coglycolide) (PLGA) microbubbles having very less negative zeta potential, 2 to 20 μm diameter ranged particles and opsonization of the surface. In 2006, Böhmeret al. developed a new method to create 4-5μm diameter ranged microbubbles using ink- jet printing. The mono dispersible microbubbles were formulated using copolymer polyperfluorooctyloxycaronyl-poly (lactic acid) [PLAPFO] incorporated into an organic phase and subsequent injection in an aqueous phase [31,32].
Polyelectrolyte multilayer shells are preparing by charging surfactant and protein layer. The oppositely charged polyions are absorbed surfactant protein bilayer. Shchukinet alwas first discovered polyelectrolyticmicrobubble shells. They used the polymers poly allylamine hydrochloride (PAH) and poly styrene sulfonate (PSS) for the polygon pair. Remarkable stability and uniform poly electric multilayer deposition were observed within the microbubbles [33,34].
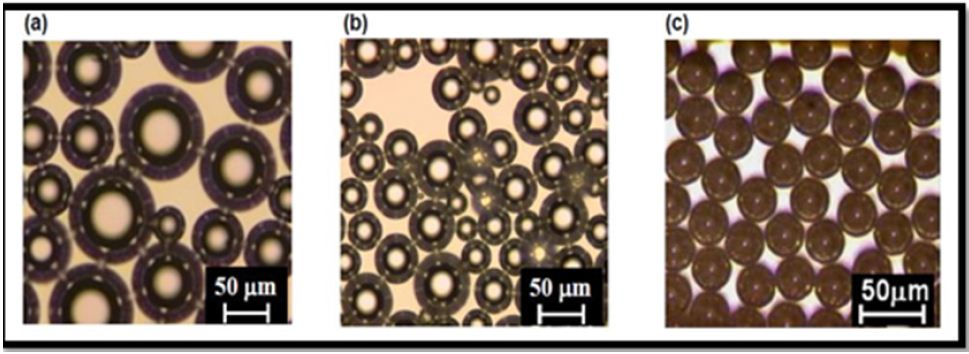
Using mechanical agitation, ultrasonication, pressurized gas-liquid mixing system, wide ranges of microbubbles can able to prepare. It is very important to prepare microbubbles with a narrow size distribution. In recent time themicrofluidic technique is vividly used for preparing monodispersemicrobubbles (Figure 2). The various other methods are also been introduced to prepare narrow sized microparticles, such as cross-linked polymerization, emulsion solvent evaporation, atomization, and reconstitution. In cross-linked polymer system, the polymeric solution is vigorously stirred resulting in the formation of colloidal stabilizer and a bubble coating agents the polymers were then cross-linked and linked on the surface of newly generated bubbles, these bubbles were then dialyzed against Milli-Q water. As for example, Using Ultra Turrax T-25 at 8000 rpm, Telechelic PVA (2% aqueous solution) was vigorously stirred for 3 hours, maintaining pH around 2.5. With the help of Teflon-coated tip, the fine form of PVA is formed. This PVA is then cross-linked at 5o C by adding HCL and H2SO4 as a crystal. After cross-linking reaction ends, microbubbles are been separated.
Figure 2: Difference of bubble size and shape when Microbubbles are prepared using (a) Mechanical agitation; (b) Sonication; (c) Microchannel emulsification.
In emulsion solvent evaporation method two-phase solutions are used. In first aqueous solution gelatin, collagen, albumin, globulins are mixed, which are actually amphilic biopolymers. These become the outer continuous phase of the emulsification system. The second phase of the mixture is prepared using in a mixture of two water-immiscible organic liquids. One liquid system must be volatile and another must be on–volatile for the polymer system. The second is made from the dissolution of a wall forming a polymer in a mixture of two water-immiscible organic liquids. One of the organic liquids is a relatively volatile solvent for the polymer & the other is a relatively nonvolatile non solvent for the polymer. The polymer solution is added to the aqueous solution with agitation to form an emulsion. The emulsification step is carried out until the inner phase droplets are in the desired size spectrum. It is the droplet size that will determine the size of the micro bubble.
In atomization and reconstitution method a spray dried surfactant solution is formulated by atomizing a surfactant solution into a heated gas this result in the formation of porous spheres of the surfactant solution with the primary modifier gas enclosed in it. These porous spheres are then packaged in a vial; the headspace of the vial is then filled with the second gas or gas osmotic agent. The vial is then sealed, at the time of use it is reconstituted with a sterile saline solution. Upon reconstitution, the primary modifier gas diffuses out & the secondary gas diffuses in, resulting in size reduction. The micro bubbles so formed remain suspended in the saline solution & are then administered to the patient.
Microbubbles diameter and size distribution are studying by using laser light scattering, scanning electron microscopy, Transmission electron microscopy. The shell thickness can be characterized by coating the shells with fluorescence dye like Red Nile; this is then determined by fluorescence microscopy against adark background. By using Colter counter method microbubble concentration can be determined. The air entrapment was studied using U-Tube densitometry with DMA-58. The instrument is calibrated with purified water and air prior to use. 5-minute high power sonication is needed for complete removal of encapsulated air.
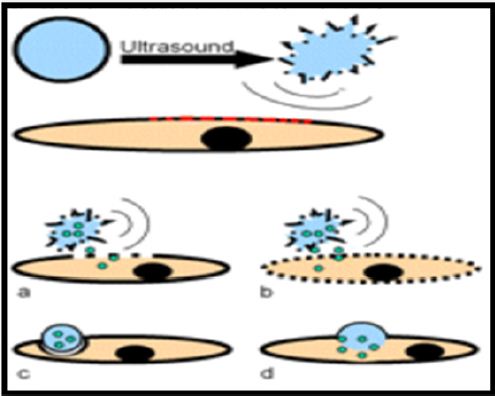
i. Microbubbles responses nicely on ultrasound. After ultrasound administration on micro bubbles, its resonates to expand and shrink. During this phenomena,microbubblescome out from lipid or protein or cross-linked polymer monolayers. During this process, microbubbles become more effective towards tissue permeation and vesicular absorption and also increase grayscale images and flow-mediated Doppler signal. Due to the harmonic signals produces by ultrasound selectively micro bubbles getcaptured (Figure 3). This process makes it more acceptable for imagining and emerging therapeutic application [31-33].
Figure 3: Drug delivery via micro bubbles- (a) Drug delivery by capitation; (b) Drug release by capitation and increasing the permeability of cell membrane; (c) Phagocytosis of the micro bubble; (d) Fusion of micro bubble with the cell membrane.
ii. Sincemicro bubbles are extremely sensitive towards ultrasound; hence it has a vital use in cavitation imaging, in nuclear medicine delivery system, optical imaging, Expansion of MRI, computed topography and Modern X-technique.
iii. Selective cells targeting and imaging of blood flow is also possible by ultrasound contrast agents. Itprovidesthe best alternative for molecular imaging. In this case, living cells can be targeted to exploit certain diseases. Microbubbles can get engulfed or during phagocytosis its get digested by lymphocytes and leukocytes. The presence of phosphatidylserine in microbubble coating materials makes it more susceptible towards engulfment by lymphocytes and leukocytes. Microbubblestargeted white blood corpuscles can use to image inflammation. Lindner has insisted that microbubbles carried white blood corpuscles would enhance ischemic lesions and reperfusion. Furthermore, molecular imaging is also possible with ultrasoundcontrast agents when microbubbles were grafted with suitable ligands. To target vasculature micro size bubbles the best Perfluorocarbon (PFC) emulsion, can be used which has an arrow diameter, which is in the range of 100nm diameter and potentially even smaller [34-37].
iv. Blood-brain barrier targeting
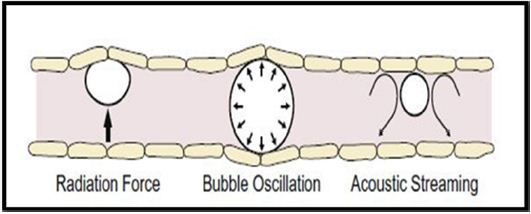
The drug administration through the blood brain barrier carried away using passive diffusion. Microbubbles and Transcranial application of ultrasound can relatively use to open blood-brain barrier. In clinical trials BR55 molecule was introduced, which has a conjugation with vascular endothelial growth factor- 2 (VEGFR2) binding peptide are admixed with peptide free phospholipids to form micro bubbles (Figure 4) [38-41].
Figure 4: Possible mechanism for Blood Brain Barrier Disruption via Ultrasound + Micro bubble.
With non-invasive technique and unique ability to penetrate biological tissues to deliver drugs, genes, ligands, and microparticles with the help of ultrasonication method, microbubble delivery system slowly becoming a promising alternative for Solid Lipid NanoParticles. These techniques have potential importance for improving drug delivery towards brain cells. New research with chemotherapeutic drugs, antibodies, and other agents into the brain cell targeting has taken its momentum. Progress has been made in understanding physical and physicochemical mechanism on ultrasound-mediated BBB openings. Continuous effort to understanding the actual mechanism and more clinical trials along with improving stability profile can boost the widespread adoption of this technology. More research is needed to create nano-sized globules to improve passive absorption and active transport within the BBB junction.


