Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Bhupendra Prajapati1*, Manan Panchal1 and Umang Varia2
Received: July 06, 2017; Published: July 12, 2017
Corresponding author: Bhupendra Prajapati, Department of Pharmaceutics, S.K. Patel college of Pharmaceutical Education and Research, Ganpat University, Ganpat Vidyanagar Mehsana-Gozaria Highway, Mehsana-384012, Gujarat, India
DOI: 10.26717/BJSTR.2017.01.000184
The main objective of this work was to examine the capacity of a nano emulsion formulation for topical application of Acyclovir. Various o/w Nano emulsion were prepared by using drop by drop addition method. By constructing pseudo phase ternary diagram Nano emulsion area was identified. The optimized formulation of Nanoemulsions were subjected to thermodynamic stability tests. After stability study, stable formulation were characterized for droplet size, polydispersity index and viscosity. Using Franz Diffusion cell and abdominal skin of rat, permeation of Acyclovir was determined. After 8 hours, Formulation NEG1 shows 90.98 % of drug permeation through the rat abdominal skin and the marketed cream shows 50.59 % of drug permeation. 50 % of the drug permeation through the skin was shows after the 3 hours which in formulation NEG1 shows the increase in permeation rat abdominal skin layers as compare the marketed cream was probably due to the mean size of internal phase droplets, which were significantly smaller in Nanoemulsions. Nanoemulsion is a novel and commercially feasible approach to improve the Topical delivery of Acyclovir and has potential to increasing the skin permeability, improving stability.
Keywords: Acyclovir; Topical delivery; Anti-viral effects; Spontaneous emulsification method
Herpes simplex virus (HSV) is a double-stranded, enveloped, DNA virus. Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) belong to the family Herpesviridae, subfamily Alpha herpesvirinae. Following initial infection, the herpes viruses become latent in the sensory neural ganglia (the trigeminal ganglion in HSV-1 infection [1] and the sacral ganglion in HSV-2 infection).ACV mainly used for the first line treatment of herpes virus infection., and varicellazoster virus (VZV).The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. ACV has low oral bioavailability of 10- 20%. ACV administered by the oral, topical, & intravenous route in herpes infection [2] When administrated orally often results in general side effects, including Anaphylaxis, angioedema, fever, headache, pain, peripheral edema, gastrointestinal disturbances likes diarrhoea, nausea [3]. Topical administration of ACV (cream or ointment) has side effects like flakiness of the skin, burning or stinging feeling, or itching of skin [4]. Controlled drug delivery by topical administration produce steady-state phenomenon which is ultimately reduced systemic side effect and provide improved efficacy as compared to other dosage forms. Main disadvantage of Acyclovir is that it is poor water solubility and it is insoluble in hydrophobic solvents. So, it is not possible to produce a topical formulation which having sufficient concentration of active ingredient to produce its desired therapeutic effect and it is also difficult to optimize flux of the formulation through the skin. In case of rate of release of drug, it is also significant that any dosage form of a therapeutically active ingredient; should be stable for longer period; its potency should be retain; should not produce any colour change or produce insoluble ingredient and should also non irritating to the mucosa [5].
Acyclovir was gifted from Acme Pharma, Ahmedabad, India. Capmul MCM was gifted sample from Abitec corp., USA. Sefsol 218, Sefsol228, PDD was gifted sample from Nikko chemicals, Japan. Labrafac gifted sample from Gattefosse, France. Acrysol k 150 and Acrysol el-135 gifted from Corel Pharma chem. Ltd, Ahmedabad, India. Tween 80, glycerol, PEG 400and Carbopol 934 P procured from S.D. Fine-chem. Ltd., Mumbai, India. In this project all solvents and chemical used were of analytical Reagent grade.
Standard stock solution of drug prepared by, transferring 10 mg drug in 100 ml volumetric flask, using double distilled water for dissolving and volume make up. In double distilled water Standard solution of 100μg/ml was prepared and series of 2μg, 4μg, 6μg, 8μg, 10μg, 12 μg, and 16μg concentration solutions were prepared. The absorbance of the solutions was measured by UV visible spectrophotometer at 253 nm against double distilled water as a blank and calibration curve was constructed [6].
Solubility of Acyclovir: The solubility of Acyclovir in different oils (Olive Oil, Sefsol 218, Sefsol 228, PDD, Capmul MCM, and Labrafac), surfactants (Tween 80, Acrysol k 150, Acrysol EL 135, Acrysol k 380) and co-surfactants (Glycerol, PEG 400, Ethanol) was determined by Acyclovir, 100 mg was added to 2 ml of the selected co-surfactant, surfactants and oils in 2ml plastic appendrop. This mixture was mixed on the vibro-mixer on touch mode for 1-2 hours, mixture than allow to stand for 2 hours & centrifuge for 15 min. at 3000 rpm .From this centrifuge mixture supernatant was separated & 1ml sample was diluted with the 9 ml of double distilled water to yield a fine dispersion [7]. The resulting dispersion was assessed spectrophotometrically by measuring the absorbance value of the dispersion at 253 nm, using double distilled water as blank.
Pseudo Ternary phase diagram: Using Sigma plot version 11.0 software ternary phase diagram of nanoemulsion were prepared to decide the nanoemulsion zone in which at any point, nanoemulsion can be prepared. The surfactant (Tween 80, Acrysol K150, Acrysol EL 135, Acrysol K 180) and Co-surfactant (Glycerol, PEG-400) were selected in the ratio of 1:1, 3:1, 5:1, 9:1. The nanoemulsion were prepared by increasing the concentration of surfactant/Co-surfactant from 10% to 90% with respect to decreasing the oil phase (Capmul MCM) concentration from 90% to 10% to ascertain the highest uptake of water by nanoemulsion up to which it remain translucent. Different ratio for oil: surfactant/co surfactant ( 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9) were selected. On the basis of screening data for optimization following surfactant & co surfactants were selected for ternary phase data studies (Table 1).
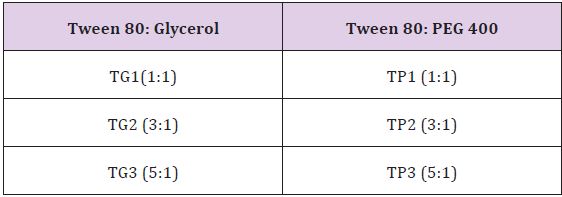
Table 1: Different Concentration. Of Surfactant /Co surfactant For the Ternary Phase Diagram Studies.
From all ternary phase diagram developed, different batch were selected from the nanoemulsion zone so that the Acyclovir could be fused into the oil phase. For each selected batches Acyclovir concentration 0.2 % (w/w) was kept constant.
Different formulations of nanoemulsion were prepared using the drop by drop addition method.1-4 First oil and surfactants / co surfactants were mixed together & stir for the at least 15 min. After that addition of the drug in the mixture & again stir for 10 min. aqueous phase taken as continuous phase, so prepared oil phase was slowly added into the aqueous phase & stir for 20 min. Finally nanoemulsions were prepared (Table 2).
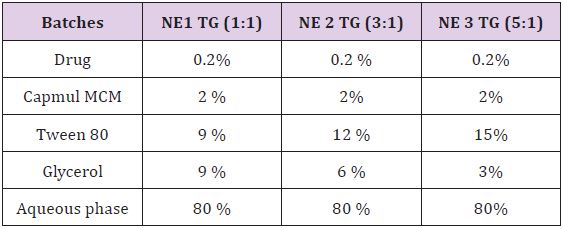
Table 2: Batches Selected from the Ternary Phase Diagram investigation.
In double distilled water acceptable quantity of Carbopol-934 P was dispersed to form Nanoemulsion gel (NEG1). For the exhaustive swelling of Carbopol-940dispersion was kept in dark for 24 h. Acyclovir was dissolved in Capmul MCM. Acyclovir solution was added slowly to Carbopol-940 dispersion. Triethanolamine was added in this mixture to counterbalance Carbopol-940.Then mixture of Tween 80 and Glycerol (1:1) were added slowly. To form a final preparation remaining amount of double distilled water was added [8] (Table 3).
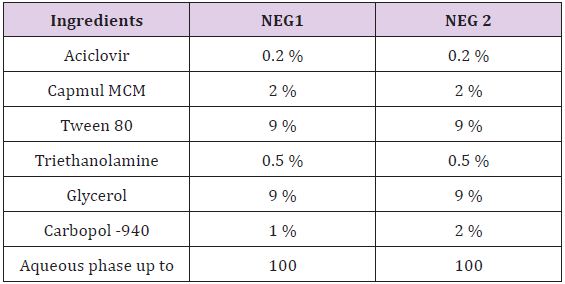
Table 3: Composition of nanoemulsion gel.
For the measurement of particle size and its distribution, A Malvern laser light scattering masterizer model 4700 equipped with and argon laser was employed. Scattering of light was supervised at 90° angle and 25°C. By assuming Spherical particles, mean droplet size and Polydispersity index were calculated from bimodal distribution, intensity, and volume.
For the measurement of zeta potential, Zetasizer HSA 3000 was used. Before each experiment cuvettes were cleaned with methanol and washed by samples which were to be analyzed and results were noted down [9, 10].
Prepared formulations were subjected to measurement of viscosity using Brookfield viscometer DV-II+ Pro model. Sample adaptor of Brookfield Viscometer contained Specific formulations and viscosity was determined at 250C. The determination was carried out by using spindle at the speed of 100 RPM and after 10 minutes of the spindle rotation viscosity was determined. The determination of viscosity was made in triplicate and each time fresh sample was poured.
The pH of the prepared formulations was measured using Systronic, 361-micro pH meter at 25 ±1ºC.
A stability study was performed by introducing them to accelerated temperature stability and centrifugation. According to ICH Q1(C) guideline, formulations sample kept at different temperatures (2-8°C, 250C) for one months and visual inspection was carried out by observing samples after month & evaluated for parameters like phase separation, flocculation & precipitation. Centrifugation of formulation in mini spin centrifuge at 1000 rpm for the 15 minutes. Prepared nanoemulsion was evaluated for flocculation, creaming, and oil separation using ultracentrifuge.
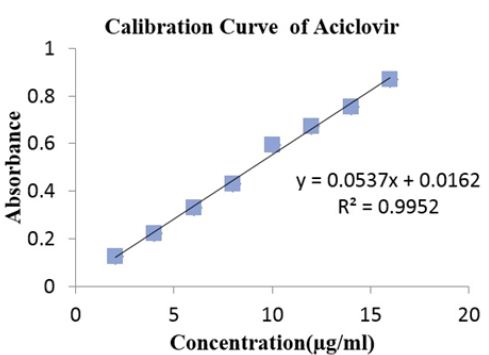
Figure 1:Calibration Curve of Aciclovir at 253 nm.
The maximum solubility of Aciclovir was found in Acrysol EL- 135 and Tween 80 as compared to any other surfactants. Tween 80 reported a virucidal activity thus is selected for the formulation. The highest solubility of ACV was found in glycerol. Therefore Tween 80 and Glycerol were selected as surfactant and co surfactants respectively for the phase study. Aciclovir was highly soluble in Capmul MCM as compared to any other oils (Figures 2A-2C).
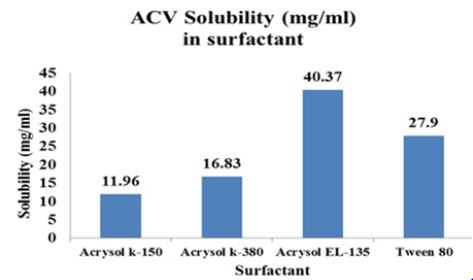
Figure 2A:Solubility of drug in surfactants.
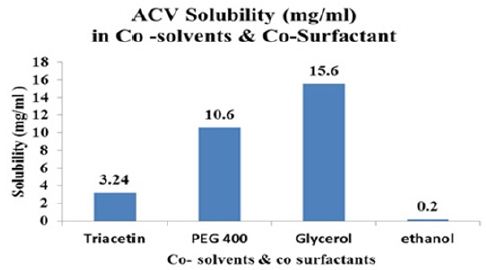
Figure 2B:Solubility of drug in co-solvents & cosurfactants.
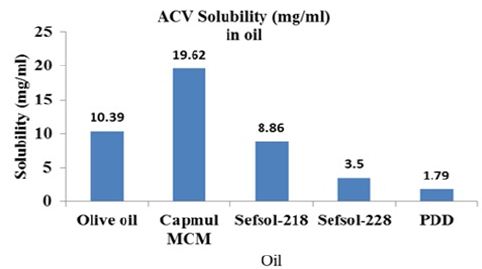
Figure 2C:Solubility of drug in various oil.
For the identification of oil in water nanoemulsion regions and optimization of formulations, ternary Phase diagrams were constructed separately for all Smix ratio. In (Figure 2C), large nanoemulsion region was found in Smix with a ratio 1:0.In the ternary phase diagram, the region where highest amount of water present, O/W nanoemulsion was found in that region and Smix which was in higher concentration, exhibit that Tween 80 could be used alone without Capmul MCM, but it would only possible when the concentration of surfactant would be higher. By using approximately 15% (m/m) of Smix, highest concentration of surfactant could be dissolved in the ternary phase diagram. The interfacial film became less viscous and liquid crystalline region was found to be absent in ternary phase diagram when cosurfactant and surfactant was combined, which was observed in 1:1 Smix. It was examined that highest amount of oil that could be dissolved was 15% (m/m) with around 30% (m/m) of Smix. Higher nanoemulsion area was discovered, as the surfactant concentration was rised in Smix (ratio 1:1). In Smix as we caused further increment in concentration of surfactant from 1:1 to 2:1 there was reduction in nanoemulsion region was found as compared to 1:1. Same observation was found when co surfactant concentration increased .The highest concentration of oil that could be solubilized by this ratio was 15% (m/m) utilizing 30% (m/m) of Smix. As the surfactant concentration increase viscosity of the formulation was increases. So, higher viscous nanoemulsion may not suitable for the formulation. From the ternary phase diagram (A), (B) & (C) the surfactant: co-surfactant (1:1), oil: surfactant (1:9) figure 3 (C) ratio shows transparent formulation with desirable viscosity (Figures 3A-3C).
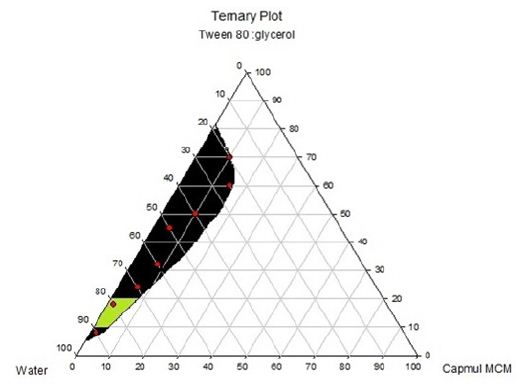
Figure 3A:Solubility of drug in surfactants.
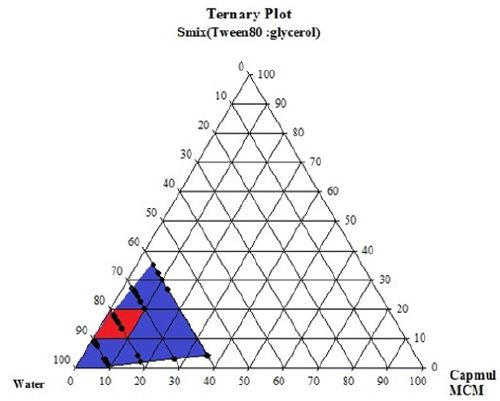
Figure 3B:Solubility of drug in co-solvents & cosurfactants.
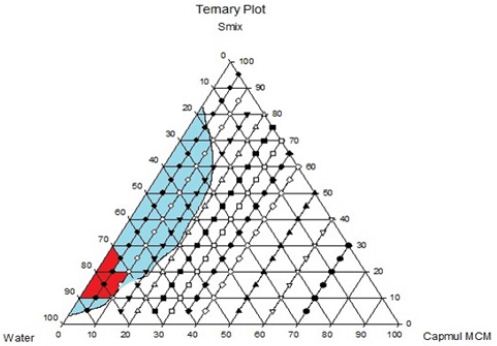
Figure 3C:Solubility of drug in various oil.
The average droplet size of nanoemulsion was found to be 10 to 100 nm. As oil droplets have capacity to fuse with the skin and thus producing a pathway for drug delivery, for that a small droplet size are mandatory. For the measurement of particle homogeneity Polydispersity index (PI) is prerequisite and it fluctuate from 0.0 to 1.0.As the value of Polydispersity index (PI) is near to Zero it indicate that particles are more homogeneous [11,12]. Formulations showed their PIin the range of 0.523 to 0.835 that expressed adequate homogeneity. Zeta potential value of all Nanoemulsion batches was found in the range of -40.3 to -15.7 mV (Table 4).
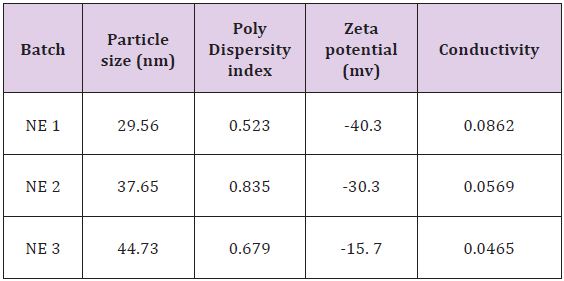
Table 4:Solubility of drug in various oil.
Formulations NEG1 & NEG2 shows pH ranges between 6.5 - 6.9 which shows gel formulations had pH ranges near to the neutral pH which may not produce any irritation to the skin. Formulation NEG1 has viscosity of 6.9± 1.26, which is nearer to neutral pH & may not produce irritation to the skin. Spreadability of topical formulation is the one of the main criteria to be considered for the stability &uniformity of dose. Formulation NEG1 contain 1% Carbopol 934 P has spreadability of 10.75± 0.532 (g.cm/sec) & viscosity 1164 ± 1.329 (cps), and NEG2 formulation contain 2% Carbopol 934 P has spreadability of 11.28± 0.743 (g.cm/sec) & viscosity 1673± 1.108 (cps), from both formulation NEG2 shows higher spreadability but higher viscosity makes it more consistence (Table 5).
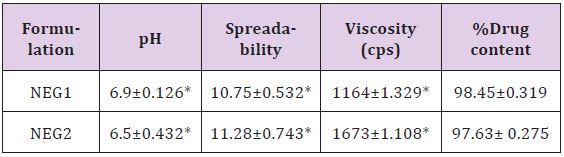
Table 5:Evaluation of Nanoemulsion Gel.
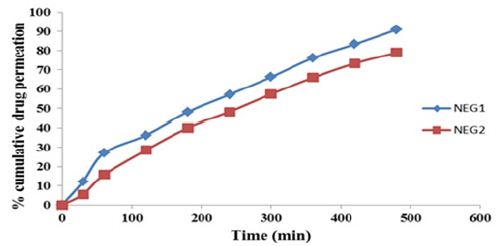
Figure 4:Solubility of drug in various oil.
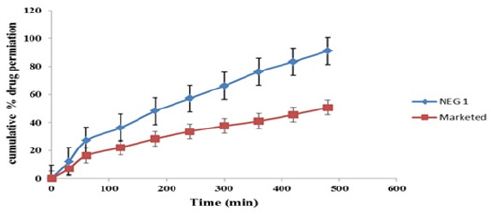
Figure 5:Solubility of drug in various oil.
From the constructed phase diagram the ratio of oil (Capmul MCM), surfactant/co surfactant (Tween 80/ Glycerol) & distilled water topical nanoemulsion can be formulated & it can be the suitable novel alternative to the emulsion for improve the topical delivery of less permeable drugs like Acyclovir and has potential to increase skin permeability, improving the stability, non-irritant to skin & cost effective final formulation of Acyclovir [13-19].
We are thankful to Acme Pharma for kindly supply the drug. We are also thankful to, Corel Pharma and Gattefossae,Francefor kindlysupplytheAcrysol and Labrafac.


