Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Krishna Kumar Prajapati and Ravi Kant Upadhayay*
Received: June 06, 2017; Published: June 19, 2017
Corresponding author: Ravi Kant Upadhayay, Deen Dayal Upadhayaya Gorakhpur University Gorakhpur-273009, UP, India
DOI: 10.26717/BJSTR.2017.01.000141
Yellow wasp Polistes flavus venom toxins were isolated by cutting the last two segments of abdominal region and purified on Sepharose CL- 6B 200 column, the molecular weight of venom protein from yellow wasp ranging from 6-70kD. The purified venom toxins were investigated for its antimicrobial activities against three infectious bacterial pathogens. Paper disc diffusion and serial dilution assays were performed for determination of Inhibition Zone Diameter (IZD) and Minimal Inhibitory Concentration (MIC) respectively. MIC values obtained were 12.3 μg/ ml against E. coli, and 24.69 μg/ml for Salmonella typhi and Vibrio cholera. By agar disc diffusion method, the Inhibition Zone Diameter (IZD) for E. coli, Salmonella typhi and Vibrio cholera were 36±0.14, 19.96±0.36 and 21.69±0.22 respectively. In present investigation, Triton X-100 was found good solubilizing agent for venom protein. The antibacterial effects of wasp venom may be due to the action on cell membrane destruction and cell-lyses.
Keywords: Polistes flavus; Venom protein; Inhibition Zone Diameter; Antibacterial activities and peptides
Abbreviations: AMP: AntiMicrobial peptides; PCD: Programmed Cell Death; PDVs: PolyDnaViruses
Hymenopteran insects mainly wasps, honey bee, hornets inflicts venom to maintain self-defense for protection of territory. Hymenopteran venoms are secreted from poison glands which are attached to a sting apparatus present in last abdominal segment. It serves both as defensive substances against aggressors as well as weapon used to paralyze the victim during gaining food. Wasps react and respond very fast to make an attack on predators and mammals. It is also highly toxic to micro-organisms. Venom toxins have been evolved to capture prey and make defense against predators and/or micro-organisms. Generally, wasp envenomation occurs after little disturbance near the hive, they inflict venom into the body of mammals mainly man and his pets [1]. Wasps inflict venom for making self defense. Chemically the venom protein of wasp is the mixture of biologically active substances of highmedium and small molecular weight with a variety of physiological function [2].
Wasp venom is a complex mixture of several hundreds of different components and lethal toxins. It is the complex mixture of active amines such as serotonin, histamine, tyramine, dopamine, etc and peptides such as kinins, mastoparans, crobroline etc. and many types of enzymes such as hydrolases (proteases, hyaluronidase, phospholipases, phospholipases A, nucleiotidases) and as well as allergens [3] and toxins [4]. Wasp venom is highly toxic to small mammals in which it causes tissue irritation, swelling, redness, inflammation and pain. The wasp toxin induce severe anaphylaxis in animals and man soon after stinging [5] and cause early and delayed hypersensitivity, inflammatory reaction, necrosis and toxic complications in mammals and even invertebrates animals [6]. Venom toxins of Polybia poulista showed genotoxic and mutagenic effects. Polyamines toxins from yellow wasp is potent openchannels blockers of ionotropic glutamate receptors that shows selective ligand binding [7].
Crude venom of Polybia poulista exhibits strong antibacterial activity against both gram positive bacteria and gram negative bacteria [8]. Wasp toxin shows potent antibacterial activity and can be used as effective antimicrobials to treat infectious diseases [8]. These also show insecticidal activity in small insect lepidopteran larvae. Wasp venom shows low MICs values in micro broth kinetic system and in conventional broth micro-dilution [9]. Anoplin an antimicrobial peptides (AMPs) is a promising candidates for battling multi-resistant bacteria [10]. Anoplin is a novel analogue with high antibacterial activity and enzymatic stability, which may represents a potent agent for the treatment of infection [11]. These antibacterial peptides from wasp venom showed broad spectrum antimicrobial activity and acts through diverse mechanisms [12]. Mastoparans is α-helical and amphipathic tetradecapeptides abtained from the venom of the wasp Vespula lewisii exhibits a wide variety of biological effects, such as antibacterial activities. Mastoparans increased the level of histamine release from mast cells cytotoxicity [13].
Venom of many animals used as antibacterial agent to treat infection. Wasp Polistes flavus crude venom shows antibacterial diseases against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and two Gram-positive bacteria (Escherichia coli and Klesielia pneumonia). It could be considered a potential source for developing new antibacterial drugs [8]. Antimicrobial decapeptides anoplin shows strongly antifungal activities against plants pathogens such as Lepidophaeria maculans and shows protection of Brassica napus plants diseases [14]. In present investigation, venom from Yellow wasp Polistes flavus was isolated, purified and evaluated for its antibacterial agents against three important microbial diseases pathogens i.e., Salmonella typhi, Vibrio cholera and Escherichia coli.
Escherichia coli(ATCC 25922), Salmonella typhi(MTCC 98) and Vibrio cholera(MTCC 3906) were maintained in the laboratory in Luria broth (2% w/v) for 4 days at 37°C before use, 100μl of the overnight culture was mixed in the test as well in control. Bacterial cultures were stored at 4° C and sub cultured after 8 days in solid agar medium.

Figure 1A: Microbial culture in Luria broth medium.
Figure 1B: Sub-culture in solid agar medium.
The living wasp Polistes flavus were collected from different region of Gorakhpur city. The collected wasps were immobilized by quick freezing at -20° C. The venom gland were taken out by cutting the last two segment of abdominal region of wasp and venom gland were homogenized in phosphate buffer saline (50 mM, pH 7.2) with the help of power homogenizer. The homogenate was centrifuged at 10000 rpm at 4°C for 10 minutes and the supernatant was used as crude venom of yellow wasp (Figure 1).
The field collected yellow wasp Polistes flavus in 150 numbers were anesthetized with chloroform, and dissected in cold PBS and their sting apparatus with venom gland were taken out from its last abdominal segment. These were homogenized in glass-glass homogenizer in 5ml of different solubilizing buffer agents such as Triton X-100, TCA 10%, Tris+EDTA, PBS and Absolute alcohol separately. Homogenate was centrifuged at 10000 rpm for 10min at 4°C and supernatant was taken out and venom protein present in supernatant was estimated by the Lowry’s method [15].
Proteins were eluted on a Sepharose CL-6B-200 a double cavity gel filtration column [16] with sintered disc filtered in the bottom having a height of 1 meter in 25 mm diameter. A known volume i.e., 5ml of toxin protein solubilized in PBS was loaded in the column and the flow rate between 1 ml/minute was maintained by a continuous buffer supply in a cold room. Eluted fraction collected at a fixed time interval using a Pharmacia fraction collector and the values of protein concentration in different eluted fraction were plotted on graph; absorbance was determined at 280nm. Column was tightly held by clips and held erect with stand. The eluted fractions containing venom protein were pooled and lyophilized to a desired concentration of the venom proteins. Dialysis bag made of cellulose membrane was boiled for 10 minutes in a large volume of 2% sodium bicarbonate and 1mM EDTA and then rinsed the membrane throughly in distilled water inside and outside before use. The lyophilized venom protein was filled in the dialyzing bag and dialyzed again three changes of phosphate buffer (50 mM, pH 7.2) to remove the excess salt from the lyophilized protein venom solution of Polistes flavus (Figure 2).
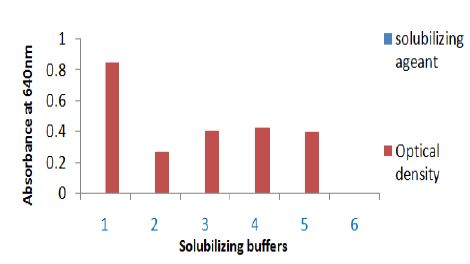
Figure 2: Solubilization of sting gland protein of Polistes flavus in different buffer. The absorbance of solubilizing protein was taken at 640 nm. Solubilizing buffer on X axis are 1. Triton X-100, 2. Phosphate buffer, 3. 10% TCA, 4. Tris+EDTA and 5. Absolute alcohol.
Wasp venom toxin was evaluated for the antimicrobial activity test by agar disc diffusion method. In this test six mm sterile filter paper discs (Whatman No. 1) were coated with different concentrations of wasp venom toxins, prepared in Phosphate buffer saline (pH 6.9). Inoculums size was adjusted to 106 colonyforming units (CFU/ml). It was spread evenly on agar plate surface by a sterile rubber pad. Each toxin was assayed in triplicate. Sterile distilled water was used as negative control. Tetracycline, Ampicillin and Ciprofloxacin were used for comparison. Plates were incubated for 24 hours at 7°C and diameter of inhibition zones were measured (NCCLS) [17].
Susceptibility tests in the liquid medium were conducted according to the method of Amsterdam’s method [18]. Wasp toxins were diluted by serial micro-dilution method (up to 10-10) using Luria Broth, final concentrations ranged from 58.57 to 0.229 mg/ ml and assays were done in triplicate. MIC values were the lowest concentration of the yellow wasp toxins where no turbidity was observed in the culture flask after 24 hours incubation at 37°C for three infectious bacterial species and it was standardized in the terms of absorbance at 600 nm in spectrophotometer. For the determination of Minimal Bactericidal Concentration (MBC) inoculums size was adjusted to 106 CFU/ml in sterile agar plates and determined again after incubation at 37°C for 24 hours in all test and control discs. The lowest concentration at which no visible growth was obtained in agar plates was considered as MBC values. For evaluation of inhibition two parallel controls were set for each test extract. Bacterial growth was observed in the presence of different quantities of wasp venom toxin as well as in its absence (Figures 3 & 4).

Figure 3: Inhibitory effects of Polistes flavus venom (T=98.56-1.92μg/ml) on the growth of different bacterial strains compared with P1-Tetracycline,P2-Amphiciline and P3-Cyprofloxacine.
Figure 4: MIC values for three infectious bacterial species by Micro-dilution method.
A. In the present investigation, Triton X-100 proved to be a good solubilizing agent for venom protein. Higher protein solubilization was observed in the supernatant than in the residue, except TCA (Figure 2). The elution pattern of purified and homogenized sting gland of yellow wasp exhibited two major peaks at 280nm in the fraction no. 41-61 and fraction no. 81-101(Figure 5A). Further concentration and fractionation of venom proteins again revealed two peaks at 640nm, a minor one between the fraction no. 46-51 and a major peak between fractions 60-101 (Figure 5B). Both peaks were eluted with 0.13M NaCl PBS buffer (pH 6.9) and protein estimation was done for each fraction by using Lowry´s method. The total yield of protein was 69.21% and specific activity was determined in each fraction (Figure 5C). The molecular weights of wasp venom protein fractions were ranging from 6-70kD (Figure 5D).
B. The MIC values were 12.3μg/ml against E. coli, for Vibrio cholera 24.69μg/ml and 24.69μg/ml for Salmonella typhi. In this investigation the result suggested that E. coli was most susceptible to yellow wasp toxin, while the Vibrio cholera and Salmonella typhi were least susceptible against yellow wasp toxin (Table 1). By agar disc diffusion method the diameter of inhibition zones in mm in presence of wasp toxin at a concentration range 98.56-6.9μg/ ml of investigation E. coli, Salmonella typhi and Vibrio cholera are 18.36±0.14mm, 19.96±0.31mm and 21.83±0.22mm respectively (Table 2).
In the present study, the antibacterial activity of purified yellow wasp venom was determined in vitro and compared with broad spectrum antibiotics. From the antibacterial susceptibility tests it was found that antibiotics were found less active than wasp venom toxins against the test bacteria. Yellow wasp venom toxin (16.4mg/ ml) gave inhibition zone diameters of 18-21mm, while tetracycline, ampicillin and ciprofloxacin gave inhibition zone diameters in the ranges of 12-16mm at a concentration of 8mg/disc. By agar disc diffusion method the diameter of inhibition zones in mm in presence of yellow wasp toxins at a concentration of 6.9-49.24 μg/ ml were 18.36±0.14mm, 19.96±0.31mm and 21.56±0.22mm for E. coli, Salmonella typhi and Vibrio cholera respectively. Inhibition zone diameters in mm for tetracycline against E. coli, Salmonella typhi and Vibrio cholera were 15.66±0.22mm, 16.43±0.28mm and 9.86±0.17mm, while for Salmonella typhi the inhibition zone diameter were 12.46±0.19mm, 13.63±0.21mm and 12.00±0.24mm. For Vibrio cholera the inhibition zone diameter were 12.06±0.22mm, 12.36±0.28mm and 13.60±0.23mm respectively.
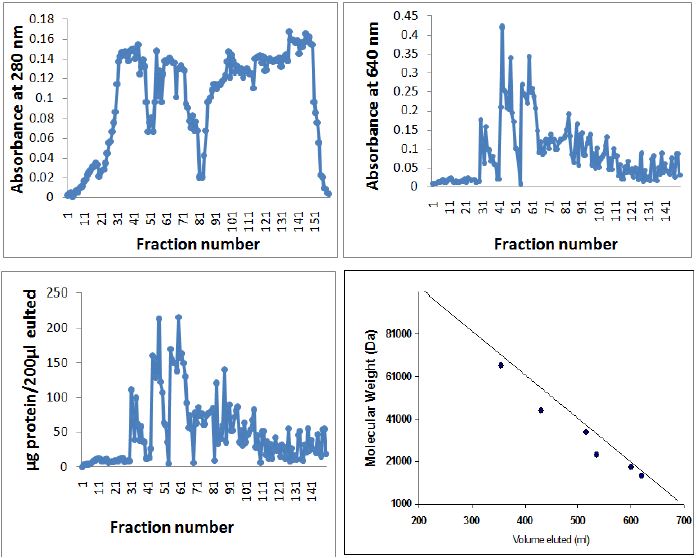
Figure 5: Elution pattern of PBS extractable protein of yellow wasp Polistes flavus chromatographed on the Sepharose CL6B 200 column. A. Absorbance at 280 nm., B. Absorbance at 640 nm., C. μg protein/μl 200 fractions by Lowry’s method. D. standard protein chromatographed on Sephrose CL 6B 200 column for determining the molecular weights of venom protein/peptides isolated from the Polistes flavus. Standard proteins used were Bovine albumin mol. Weight 66,000, egg albumin mol. Weight 45,000, pepsin mol. Weight 34,700, trypsinogen mol. Weight 24,000, β-lactoglobulin mol. Weight 18,400 and lysozyme mol. Weight 14,500. Elution volumes of unknown proteins were compared with log values on the X-axis for estimation of molecular weights.
Table 1: Antimicrobial activities of venom toxins isolated from Polistes flavus on different microbes and their corresponding MIC.
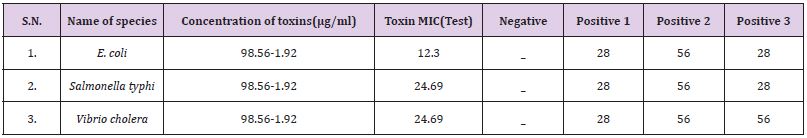
Table 2: Zone of Inhibition of wasp venom toxin isolated from Polistus flavus on different microbes and their corresponding IZD.
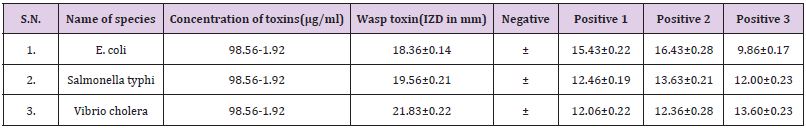
Values are expressed as mean ± SD (N=3) positive controls are 1. Tetracycline, 2.Amphiciline, 3. Ciprofloxacin, Negative control is distilled water.
In all cases, the venom toxin of yellow wasp displayed much better antimicrobial action against these bacterial stains. The results suggest that 21.83±0.22mm (Vibrio cholera) was most susceptible to yellow wasp toxins while 18.36±0.14mm (E. coli) and 19.96±0.31mm (Salmonella typhi) were least susceptible for wasp toxins. Anoplin from wasp toxins shows broad spectrum antimicrobial activity [10].especially against multi-resistant bacteria [12]. These peptides are amphipathic secondary structures with both charges and interact with anionic sites of bacterial membranes in different ways [19]. Similarly, mastoparans are α-helical amphipathic tetradecapeptides obtained found in venom of wasp Vespa lewisii.
This peptides exhibits a wide variety of biological effects, including antibacterial activity, increased histamine released from the mast cells, induction of the potent mitochondrial permeability transmission and tumor cells cytotoxicity [13]. These could be considered as potential source for developing new antibacterial drugs [8]. Antibacterial decapeptides anoplin shows strongly antifungal activity against plant pathogens Leptosphaeria maculans and shows protection of Brassica napus plants from diseases [14]. The decapeptides anoplin is smaller in small size and shows antibacterial and non-hemolytic properties. Similarly antimicrobial activity is also reported in cationic peptides Polibia-MPI [11], in synthetic analogues of anopilin [11]. Anoplin shows membrane anchoring through a lipophilic amino acid derivatives and slight improvement in biological activity [20].
Besides toxins, wasp venom also contains allergens [3,5] which induce severe anaphylactic reaction in animals and man after stinging [21]. These also act as broad spectrum antiviral drugs [22]. Wasp toxins and allergens injected in wasp stings cause severe systemic medical compilations, such as allergic reactions [23] and cause anaphylactic shock [24]. From the result it can be concluded that the purified yellow wasp toxins has displayed better and antibacterial activities against bacterial strains in comparison to the three antibiotics tested. These could replace highly toxic antibiotics which solves the problems of drug resistance. There is an urgent need to explore new drug candidates from animal toxins mainly from wasp venom against re-emerging drug resistant bacterial and viral pathogens. Venom proteins from endoparasitic wasps Nasonia vitripennis are predominately involved in regulation of host physiology and immune responses alone or in combination with other factors of maternal origin such as polydnaviruses (PDVs) or virus-like particles presents in the venom itself or produced in the ovaries and ovarians fluids [25]. Nasonia vitripennis which is parasitoids wasp has approximately 100 venom proteins. Envenomation by N. vitripennis shows developmental arrest, selective apoptosis and alterations in the lipid metabolism in flesh fly hosts [26] while, mastoperans-induced programmed cell death (PCD) in the unicellular alga Chlamydomonas reinhartii [27]. The toxins affect nervous system of invertebrates and few invertebrates-specific peptides neurotoxins that have been isolated from the venom of spiders, ants, scorpians, wasp etc. discussion their potential in the pest control and as invaluable tools in neuropharmacology [28]. The venom, along with PDVs produced in the reproductive system of this wasp, are essential for successful parasitism as they protect the parasitoid from encapsulation by the host’s immune cells [29] and interfere with the host’s nutritional physiology [30].


