Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Narges A Albibas1, Osama A Bheleel1 and Fathi M Sherif2*
Received: May 31, 2017; Published: June 15, 2017
Corresponding author: Fathi Mohamed Sherif, Department of Pharmacology and Clinical Pharmacy, University of Tripoli, P. o. Box 82 757, Tripoli, Libya, North Africa
DOI: 10.26717/BJSTR.2017.01.000135
Background: Heart failure is a major cardiovascular disease and public health problem. It is complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of the blood. Trimetazidine is a cardio-protective drug that exerts cytoprotective effects by inhibition of the mitochondrial long-chain 3-ketoacyl coenzyme A thiolase.
Aim: This study was aimed to examine effects of chronic trimetazidine administration on left ventricular function, symptoms and quality of life of patients with heart failure (secondary to ischemic or non-ischemic heart disease).
Methods: This study was conducted at Cardiology unit of Tripoli Medical Center on 46 Libyan patients (39 - 85 years, 27 men : 19 women) diagnosed with chronic heart failure due to ischemic or non-ischemic heart diseases. The patients received, in addition to the standard therapy of heart failure, 35 mg of trimetazidine twice per day for six months. Patients were evaluated for left ventricular function by echocardiography after six months of the therapy. Symptoms and quality of life were assessed by Minnesota questionnaire after six months of trimetazidine use.
Results: The mean of left ventricular ejection fraction before treatment with trimetazidine was 37.9% and after treatment was 40.6%. The mean of left ventricular end diastolic diameter before treatment with trimetazidine was 63.544 mm and after treatment was 62.261 mm. Trimetazidine therapy was associated with a considerable improvement in left ventricular ejection fraction (p < 0.05) and a significant decrease in left ventricular end diastolic diameter (p < 0.05). There was no improvement in symptoms and quality of life of heart failure patients on trimetazidine therapy.
Conclusion: Treatment with trimetazidine for six months with the conventional heart failure drugs improves the left ventricular function.
Keywords: Heart failure; Left ventricular; Trimetazidine; Quality of life; Libya
Abbreviations: CAD: Coronary Artery Disease; HF: Heart Failure; COPD: Chronic Obstructive Pulmonary Disease; CVD: Cardiovascular Disease; DM: Diabetes Mellitus; EF: Ejection Fraction; IHD: Ischemic Heart Disease; LV: Left Ventricular; LVEDD: Left Ventricular End Diastolic Diameter; LVEF: Left Ventricular Ejection Fraction; MI: Myocardial Infarction; NYHA: New York Heart Association; QOL: Quality of life
Heart failure (HF) is a major cardiovascular disease (CVD) and it is a common cause of morbidity and mortality. It is a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of the blood. The clinical syndrome of HF may result from disorders of the pericardium, myocardium, endocardium, heart valves, or certain metabolic abnormalities. Most of HF patients have symptoms due to impaired left ventricular (LV) myocardial function [1]. About 85% of the patients with chronic HF had an ischemic etiology [2]. The terminology used to describe HF is basically based on measurement of left ventricular ejection fraction (LVEF). The more severe the systolic dysfunction, the more the LVEF is reduced from normal and, generally, the greater the end-diastolic and end-systolic volume [3,4].
HF may be associated with a wide spectrum of LV functional abnormalities, which may range from patients with normal LV size and preserved EF to those with severe dilatation and/or markedly reduced EF [1]. LVEF is considered important in classification of patients with HF because of differing patient demographic, comorbid condition, prognosis and response to therapies [1]. The primary abnormality in HF is impairment of LV function leading to a fall in cardiac output. Activation of sympathetic nervous system may initially maintain cardiac output through an increase in myocardial contractility, heart rate and peripheral vasoconstriction. Prolonged sympathetic stimulation leads to cardiac myocyte apoptosis, hypertrophy and focal myocardial necrosis. Sodium and water retention is promoted by release of aldosterone, endothelin and antidiuretic hormone in severe HF.
Despite advances in HF management, patients with HF experience a poor prognosis and compromised quality of life (QOL). Thus, patients with HF report poorer QOL compared to patients with other cardiac conditions or without HF [5-7]. Symptom burden, from physical and emotional symptoms, contributes substantially to poorer QOL. In HF patients, symptom status is one of the most important factors associated with adverse outcomes [8-10]. There are a variety of factors positively or negatively affecting QOL of patients which include physical, psychological, economic, social, spiritual and behavioral [11]. The goals of treatment are to relieve symptoms and signs, improve QOL, survival rate and prevent or reduce hospital admissions which are important issues for patients and healthcare system [12].
Patient education on discharge may improve patient’s QOL, reduce rate of hospital admission, and lower the cost and mortality rate [13]. Social support for patient reduces stress, hospitalization rate, mortality risk and promote treatment adherence [14,15]. Trimetazidine is a cytoprotective drug that normalizes metabolic disturbances in low-flow ischemia that is used for angina pectoris. It selectively inhibits long-chain 3-ketoacyl coenzyme A thiolase activity [16] and exerts cardio-protective effects by preservation of intracellular levels of ATP [17] by reducing calcium overload and free radical-induced injury [18]. Thus, inhibiting cell apoptosis [19,20], improving endothelial function and inhibiting cardiac fibrosis [21]. It increases utilization of glucose and lactates, which are more efficient fuels for aerobic respiration, the oxygen consumption efficiency of the myocardium can be improved [22].
A previous study (randomized controlled trials) included 955 HF patients who used trimetazidine indicated that trimetazidine is associated with a significant improvement in LVEF in patients with ischemic and non-ischemic HF [23]. The LV end-systolic volume is considerably reduced and NYHA classification is improved. Trimetazidine has a protective effect for all-cause mortality and CV events and hospitalization [23]. An additional use of trimetazidine in HF patients may decrease hospitalization for cardiac causes, improve clinical symptoms and cardiac function, thus, simultaneously improve LV remodeling [24]. This study was intended to explore efficacy and potency of trimetazidine in Libyan patients with HF and the significant importance of trimetazidine in improving LVF and QOL of the patients.
This study was conducted at Tripoli Medical Center (TMC, University Hospital, University of Tripoli, Tripoli, Libya) on patients registered and followed up at Department of Cardiology in a period of January, 2015 to June, 2016. It included 46 patients aged from 39 to 85 years old and who are known and confirmed as chronic HF patient due to ischemic or non-ischemic heart disease. The patients were 27 men and 19 women. Some patients have hypertension, diabetes mellitus (DM), hypothyroidism, bronchial asthma and/ or chronic obstructive pulmonary disease (COPD). All patients were clinically stable (NYHA classes I and II) and were on regular conventional HF therapy (beta-blockers, ACEI, ARBs, diuretics, aldosterone receptor antagonists). Some patients received antiplatelet, statins, digoxin, ivabradine and/or nitrates. 41 patients known with ischemic heart disease and had coronary angiography and 5 patients without ischemic heart disease. All patients of this study were LVEF ≤ 45% and LVEDD ≤ 83 mm. The exclusion criteria are: patients with atrial fibrillation, chronic kidney disease, acute myocardial infarction and patients on oral anticoagulants.
Patients individually underwent personal interview by the researcher after taken into consideration ethics in research and data collection (all patients were personally informed, agreed and signed about the study). This study was permitted by University of Tripoli and Tripoli Medical Center (UOT & TMC 2015). Patients received in addition to the standard therapy of HF, trimetazidine in a dose of 35 mg twice a day for six months. Patients were followed up and evaluated for LVEF and LVEDD at the beginning and six months after therapy by echocardiography. The measurement of the LV parameters (EF and EDD) were conducted by using General electric® Echo machines Vivid 7 and Teichholz method of measuring LVF in parasternal long axis in M-mode was chosen as the method of calculation in this study. Symptoms and QOL were assessed by using Minnesota questionnaire [24]. The questionnaire is divided into four major categories: physical symptoms assessed by six questions about his/her HF disease, physical & social activities assessed by eight questions, financial & social problems assessed by two questions and mental & psychological problems assessed by four questions. Minnesota questionnaire is a valid instrument in assessment of QOL of patients with HF [25].
Data are expressed as frequency and percentage while others as mean ± SD. Comparative analysis by paired t-test and one sample t- test was used whenever necessary using SPSS (version 16). A value of p < 0.05 was considered statistically significant.
Forty six patients with known history of chronic heart failure are included in this study ageing 62.02 ± 10.50 years (mean ± SD) and ranging from 39 to 85 years. More male patients (62.89 ± 10.91, n = 27) than female patients (60.79 ± 10.04, n = 11) in a ratio of 1.42: 1.00 but no significant difference between both sexes. Hypertension and DM were most common diseases in these patients but without any statistical significant difference between male and female patients. IHD was more in men than in women in a ratio of 1.5 : 1.0. In this study, about 45% of the patients had IHD, DM and hypertension and only 20% had both DM and IHD.
In Table 1, the average of LVEF of the studied patients before and after trimetazidine administration is shown. For all the patients, LVEF means before and after therapy with trimetazidine were 37.9% and 40.6% with duration of 14% - 45% and 14% - 62%, respectively. Regarding sex- and age- related differences, the average of LVEF before and after treatment with trimetazidine was 36.7% and 43% for the sex-related difference group and 36.7% and 41.5% for age-related difference group (Table 1).
Table 1: Left ventricular ejection fraction of the heart failure patients before and after treatment with 35 mg trimetazidine for six months.
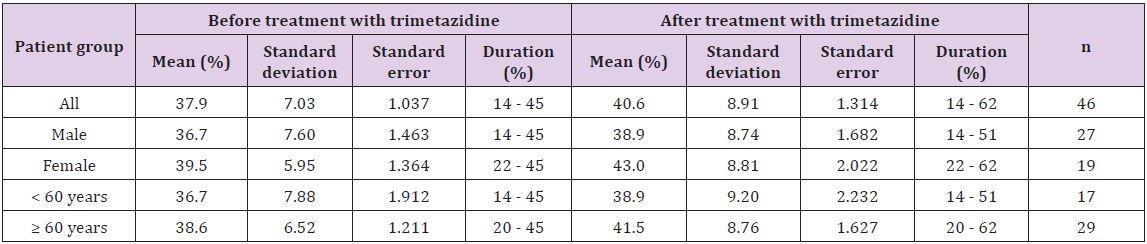
Table 2: Left ventricular end diastolic diameter of the heart failure patients before and after treatment with 35 mg trimetazidine for six months.
In Table 2, the average of LVEDD of the studied patients before and after treatment with trimetazidine for six months is shown. Thus, LVEDD for all the patients before and after administration of trimetazidine were 63.544 mm and 62.261 mm with duration of 50 - 83 mm and 45 - 83 mm, respectively. Regarding sex- and age-related differences, the average of LVEDD before and after treatment with trimetazidine was 64.852 and 60.579 mm for sexrelated difference group and 64.647 and 61.207 mm for age-related difference group (Table 2) [26].
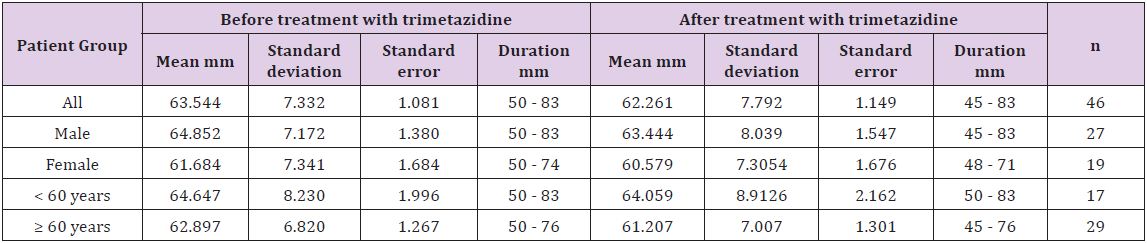
Table 3: Effects of administration of trimetazidine on left ventricular ejection fraction and left ventricular end diastolic volume of patients with heart failure.
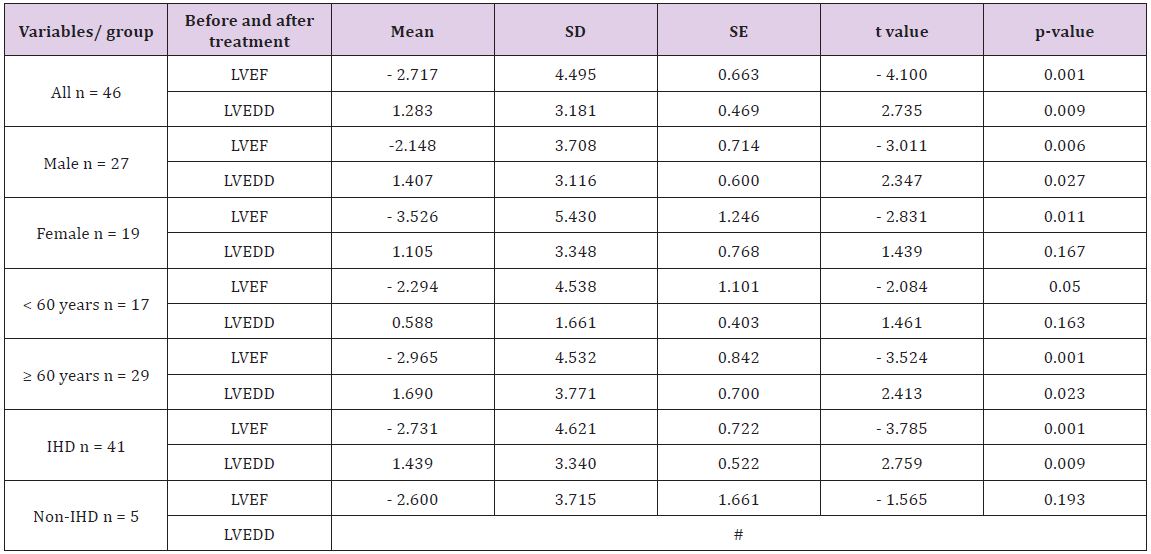
#With respect to the patients with HF due to non-ischemic cause, the statistical difference for LVEDD cannot be computed because SE is zero.
In Table 3 shows an increase in the LVFE and a decrease in LVEDD of the patients with HF. The t test reveals a highly significant increase for LVEF values (p < 0.001, t = 4.1) and a highly significant decrease for LVEDD values (p < 0.01, t = 2.74). In addition, significant changes with regard to the sex- and age-related difference were observed among the groups. There were statistical significant decreases in the LVEDD of female patients and patients who are less than 60 years old (p < 0.05, Table 3). A significant increase in LVEF with a significant decrease in LVEDD (p < 0.001 for LVEF and p < 0.01 for LVEDD) of patients with HF due to ischemic cause (IHD) with no significant change in LVEF of patients with HF due to other causes rather than ischemia (non-ischemic heart disease).
After six months of treatment with trimetazidine, the mean of LVEF of patients was significantly increased by 8% for male and 9% for female patients (from 38% to 46% for male and from 41% to 50% for female patients). The mean of LVEDD of male patients was also significantly decreased from 62.8 to 56.5 mm. However, there was no significant decrease in the mean of LVEDD of female patients (from 57 to 55.8 mm). The high percentage of patients was in age group of 55 to 64 years with the low in group aged of 34 to 44 and of patients with 85 years or older. 90% of the patients developed HF due to ischemic cause (n = 41) and only 10% of them developed HF due to non-ischemic cause (n = 5). The percentage of patients with improvement in LVEF was 33% (n = 15) which represents about one third of all the patients (n = 31) who shown no improvement.
Table 4: Symptoms and quality of life of patients with chronic heart failure using Minnesota questionnaire.
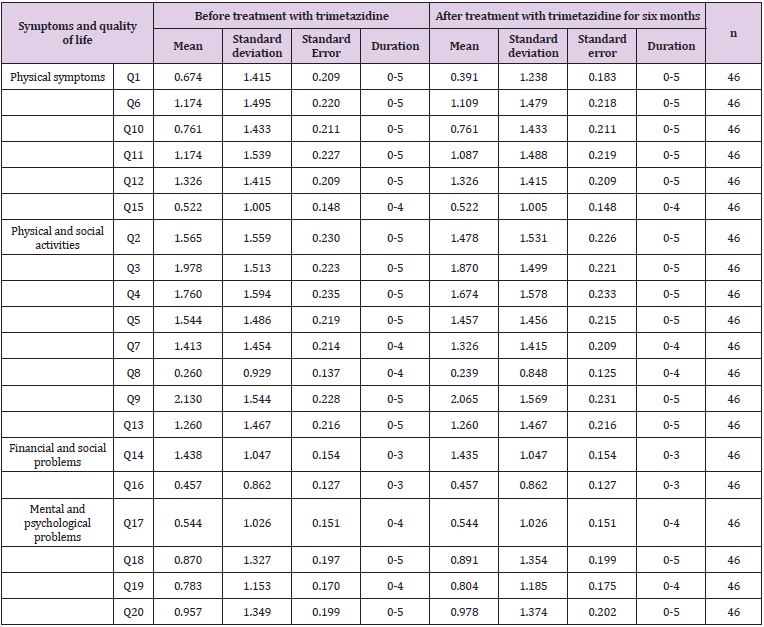
In this study, Minnesota questionnaire was used to assess the symptoms and QOL of patients with HF (Tables 4-6). In Table 4, symptoms and QOL of 46 patients assessed before and after treatment with trimetazidine are shown. Thus, no statistical significant difference in the mean symptoms and QOL of patients before and after treatment was found. The only difference found was in the mean of question number one which related to the reduction in lower limp swelling or edema. With regard to sex- and age-related differences, no significant differences in symptoms and QOL of patients were observed before and after the therapy with trimetazidine for six months (Tables 4-6).
Table 5: Effects of trimetazidine on symptoms and quality of life of failure heart patients.
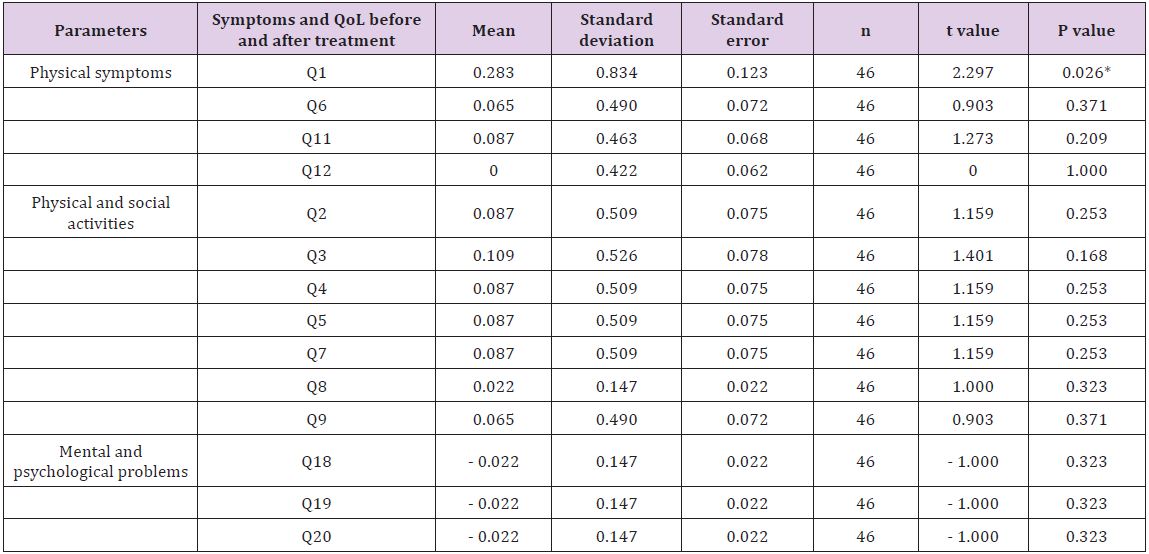
Statistical significant difference by paired Student t-test. *P < 0.05.
Table 6: Effects of trimetazidine on symptoms and quality of life of heart failure patients with ischemic heart disease.
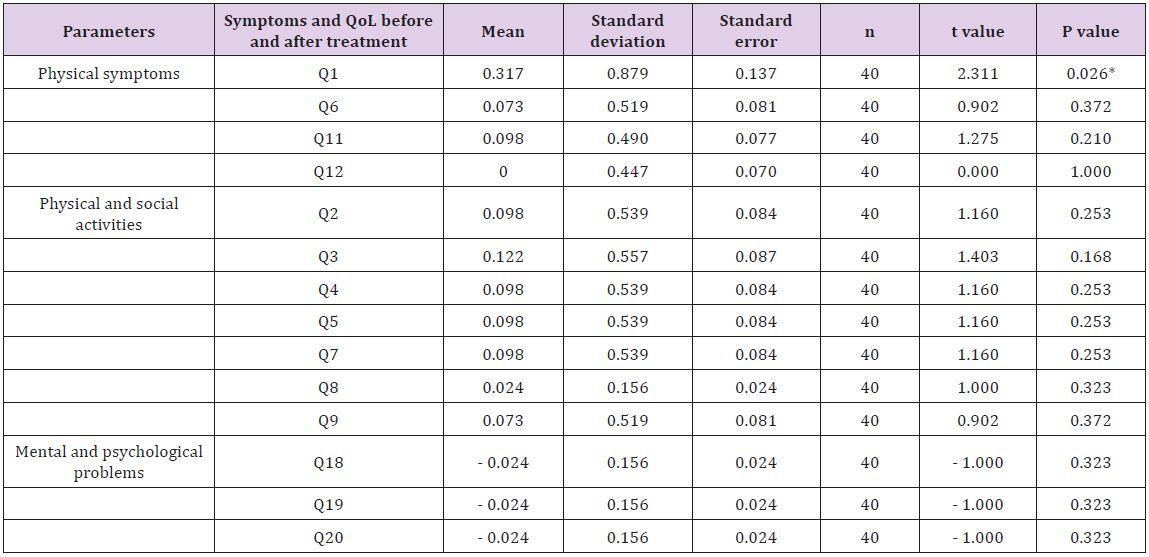
Statistical significant difference by paired Student t-test. *P < 0.05.
The treatment used in this study was recommended for patients with reduced LV function to improve their LVEF and exercise tolerance. A therapeutic intervention with trimetazidine in conjunction with the standard therapy of HF is associated a significant increase in the LVEF and an improve in the tolerance to the physical activity [27]. The major finding of chronic administration of trimetazidine to the HF patients in this study resulted in a significant increase in the LVEF and a significant decrease in the LVEDD without a change in the symptoms and QOL of the patients. This data indicate a significant increase in LVEF in one third of the studied patients and the increase in LVEF is more profound in female than in male patients.
In this study, all the HF patients were with LVEF ≤ 45% (LVEF before treatment with trimetazidine was 38%, duration: 14% - 45%). The LVEF increased to 40% (duration: 14% - 62%) after treatment with trimetazidine for six months. As shown in the present study, the LVEDD before treatment with trimetazidine was 63.544 mm (duration: 50 - 83 mm) and after treatment with trimetazidine, LVEDD reduced to 62.261 mm (duration: 45 - 83 mm). Thus, our findings indicate a significant increase in the LVEF with a profound reduction in LVEDD after treatment with trimetazidine. Regarding co-morbidities, a high incidence of patients with co-morbidities such as hypertension (56.5%), DM (69.6%) and IHD (89.1%) which are considered common risk factors for HF. Presence of comorbidities in such patients makes treatment of HF patients more complicated and difficult as a result of poly-therapy treatment, drug interactions, side effects and may worse the prognosis of HF. It is suggested that changes observed in some echocardiographic parameters (LVEF & LVEDD) after a period of chronic treatment with trimetazidine are related to the mechanism of action of the drug.
Trimetazidine considered as metabolic modulator and has an inhibitory effect on the mitochondrial long-chain 3-ketoacyl coenzyme A thiolase, which plays a critical role in the fatty acid betaoxidation pathway in the myocardium (see introduction section). This inhibitory effect leads to shifting in cardiac metabolism from fatty acid oxidation to glucose oxidation, which represents more efficient metabolic pathway in terms of oxygen consumption and energy generation (ATP production with less oxygen consumption). Also, the shifting in cardiac metabolism to glucose oxidation leads to cardio-protective effects by reducing oxidative damage in failed myocardium and decrease the remodeling [28]. About one third of the patients was improved after chronic trimetazidine (33%). Chronic trimetazidine therapy is also associated with a considerable increase in LVEF and decrease in LVEDD. This improvement in LVEF and LVEDD is related to the mechanism of action of the trimetazidine (see above). Thus, our findings are similar to other previous published studies [29-31].
Thus, a profound increase in LVEF (p < 0.001) was reported by Gunes et al. [29] who studied trimetazidine (n = 51) with an optimal HF therapy using tissue Doppler imaging after three months. Khan et al. [28] also studied 63 patients with a history of HF secondary to IHD. A group received trimetazidine (n = 31) as add on therapy to the conventional HF for three months and the other group (n =32), whom continued on conventional HF therapy without trimetazidine. All patients were assessed by echocardiographic for their LVEF and LVEDD [30]. Thus, trimetazidine resulted in significant reduction in LVEDD as compared to conventional therapy group which had increased LVEDD. LVEF improved significantly in trimetazidine group compared to the conventional group [28]. Trimetazidine is associated with significant improvement in LVEF (p < 0.01, n = 995) of the patients [23]. In a study by Hu et al. [31], trimetazidine significantly improved LVEF, with an increase of 6.88%.
Trimetazidine increased LVEF compared with conventional therapy after three months using two-dimensional echocardiography [32]. In a study by Zhao et al. [33], volunteers with DM and idiopathic dilated cardiomyopathy were recruited for participation. The patients received trimetazidine (n = 40) or placebo (n = 40) and after six months, trimetazidine treated patients had a significant improvement in systolic function. Our study showed an improvement in LVEF in women more than men with value of 42% and 26%, respectively. However, no significant improvement in LVEDD in women (p > 0.05) and in patients younger than 60 years old. With respect to the ischemic and non- IHD patients, an increase in LVEF with a decrease in LVEDD in HF patients with IHD but no improvement in LVEF and in LVEDD in non-IHD patients.
In this study, Minnesota questionnaire of QOL was divided into four major categories in order to assess the impact of therapy on QOL related to heart disease. The point regarding the sexual activities has, however, been omitted from the questionnaire for cultural issues. Data indicate no improvement in the symptoms and QOL of patients with HF, except question number one regarding the swelling in ankles or legs, (p < 0.05) which indicates a significant improvement in the lower limp swelling or edema mainly due to diuretic use. This may mean that other than drug is needed to improve QOL as intervention by education, life-style changes, etc. Our findings are in good line with previous findings reported by Marazzi et al. [34]. Thus, 47 patients (40 men and 7 women) were randomized to receive trimetazidine or placebo in addition to the standard therapy of HF for six months.
The overall assessment of QOL showed an improvement in patients received trimetazidine (p < 0.01). Fragasso et al. [32] have also assessed the LVEF (using two-dimensional echocardiography) and QOL of 44 patients with HF receiving trimetazidine in addition to full medical treatment of HF with an improvement of LVEF and QOL after three months (p < 0.0001). A cross-sectional study conducted in two University Hospitals by Saccomann et al. [33] by interviewing 170 elderly patients with chronic HF in an outpatient setting. The findings indicated a minor impact of HF illness on the emotional dimension of QOL, however, indicators related to the physical dimension, including fatigue and breathlessness, showed greater impact on QOL for elderly patients. Thus, this enabled the assessment of QOL of elderly patients living with HF, demonstrating that the physical dimension is the most compromised [35].
Morcillo et al. [36] have utilized Minnesota questionnaire to evaluate the QOL of patients with HF before and after six months of educational intervention. A total of 99 HF patients were interviewed, all of whom were admitted to hospital due to the worsening of their HF. During their stay, they completed questionnaire to determine initial scores. Those in the intervention group received an educational visit by nurse. All patients were assessed prospectively at six month. The findings indicated that Minnesota questionnaire appears to be a valid instrument in assessment of QOL. It is sensitive to changes in health since it correlates with patient prognosis [36]. The present data indicates that 75% of HF patients had financial problems (high cost of medication and medical care as repeated hospital admissions). Data also indicates that 28% of patients had loss of self-control, 37% was feeling worry, 39% had loss of concentration and 41% had depression. In Conclusion, this study indicates that chronic treatment with trimetazidine for six months with conventional HF therapy improves left ventricular function of chronic HF patients (increase LVEF with decrease LVEDD) but with no significant changes in symptoms and QOL of the chronic HF patients.
Narges A. Albibas was involved in the study design, collection and analysis of data. Osama A. Bheleel was involved in data collection, treatment and follow up of patients and Fathi M. Sherif was involved in study design and drafting manuscript. All authors read the manuscript in entirely and approved the final.
Authors wish to thank all patients who willingly participated in the survey.
The study was approved by UOT and TMC Ethics Committee.


