Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
M Amin Mir1*, Rahilla Farooq1, MMS Jassal2, Bilal Ahmad Mir3, Sandeep Kaur1 and Deepa Mishra1
Received: May 25, 2017 Published: June 07, 2017
Corresponding author: M Amin Mir, Department of Chemistry and Botany, Dehradun, India
DOI: 10.26717/BJSTR.2017.01.000118
The present work was carried out to analyze the physicochemical parameters of fixed oils of Cinnamonum zeylanicum. The oil was extracted from freshly bark of Cinnamonum zeylanicum with petroleum ether using Soxhlet apparatus. Cinnamonum zeylanicum bark possessed (47.39%) yield of absolute oil. The relative density of the fixed oil was found to be 0.96. The Physico-chemical properties of the extracted oil like Richter Meissel value (2.55 ± 0.01), Saponification value (84.15 ± 0.01), Iodine value (16.11 ± 0.02), Acid value (38.14 ± 0.05), Ester value (46.01 ± 0.08), Peroxide value (10.3 ± 0.08) and un-saponification value of (21.00 ±0.03) was determined.
Keywords Cinnamonum zeylanicum Saponification value; Iodine value; Ester value; Acid value; Iodine value; R.M. value
Awareness of medicinal plants usage is a result of the many years of struggles against illnesses due to which man learned to pursue drugs in barks, seeds, fruit bodies, and other parts of the plants. Contemporary science has acknowledged their active action, and it has included in modern pharmacotherapy a range of drugs of plant origin, known by ancient civilizations and used throughout the millennia. The knowledge of the development of ideas related to the usage of medicinal plants as well as the evolution of awareness has increased the ability of pharmacists and physicians to respond to the challenges that have emerged with the spreading of professional services in facilitation of man’s life. While the old peoples used medicinal plants primarily as simple pharmaceutical formsinfusions, decoctions and macerations in the Middle Ages, and in particular between 16th and 18th centuries, the demand for compound drugs was increasing.
The compound drugs comprised medicinal plants along with drugs of animal and plant origin. If the drug the was produced from a number of medicinal plants, rare animals, and minerals, it was highly valued and sold expensively The bark and the leaves of Cinnamonum sp are commonly used as spices in home kitchens and their distilled essential oils are used as flavoring agent in the food & beverage industries. The chemical analysis of the oils from both the sources revealed that the active oil contained 74% cinnamaldehyde, compared to only 8.3% in the inactive oil as shown by S. Elumalai, R. Kesavan, S. Ramganesh, R. Murugasen [1]. Cinnamon has been reported to have remarkable pharmacological effects in the treatment of type II diabetes and insulin resistance. Essential oils of cinnamon species have lot of biological activities including antimicrobial, antioxidant and antifungal properties.
Furthermore, cytotoxic and apoptotic activities of several constituents were identified throughout its biological properties. In the present paper, essential oil (EO) obtained by hydrodistillation of leaves of Cinnamonum zeylanicum Blume (Lauraceae) collected respectively at Cocotomey (Atlantique, Southern Benin) was analyzed using capillary GC and GC/MS, by Yehouenou Boniface, Sessou Philippe [2]. Sessou Philippe, Farougou Souaïbou, and Sohounhloué Dominique [3] was to assess in vitro antifungal activity of essential oils extracted from fresh leaves of Cinnamonum zeylanicum and Ocimum gratissimum obtained by hydro distillation. The potential of Cinnamon essential oil as a natural insecticide and ant repellent was studied by Nur Nasulhah Kasim, Syarifah Nursyimi Azlina Syed Ismail, N.D. Masdar, Fatimah Ab Hamid, W.I Nawawi [4]. Cinnamonum zeylanicum also called as Cinnamon bark is the dried inner bark of the coppiced trees, belonging to the family Lauraceae. It is considered to be native of Sri Lanka and Malabar Coast of India. It contains tannins and flavonoids which have been reported by Patel Mitul, Mukund Handral [5] to have anti-venom activity.
For the extraction of essential oil from Cinnamonum zeylanicum, solvent extraction was performed and concrete & absolute oils were obtained. 50g of Cinnamonum zeylanicum were used for the extraction of oil. The extraction process was carried out by distillation process, using water as a solvent. When the entire aroma was taken out by solvent, the distillate was concentrated by heating process to get concrete oil. The apparatus used in each process was thoroughly washed and dried.
Dissolved organic residue in the water was collected in a flask and dried over by adding anhydrous NaSO4. Concrete oil was taken in pre weighed 100ml flask and the weight of concrete oil was determined by again weighing the flask. Percentage yield of concrete oil was calculated on the basis of dry weight of the plant material taken.
Concrete oil was dissolved in minimum volume of absolute alcohol to remove the natural waxes present in the essential oil and was then filtered through a Whitman filter paper # 43. Alcohol was removed by distillation. Percentage yield of absolute oil was also calculated on the basis of dry weight.
Determination of Acid Value: The acid value is the number which expresses in 1g of the substance .Milligrams the amount of potassium hydroxide necessary to neutralize the free acids present.
Determination of Saponification Value: The saponification value is the number of milligrams of potassium hydroxide necessary to neutralize the free acids and to sponify esters present in 1g of the substance.
Determination of Ester Value: The Ester value is the number of milligrams of potassium hydroxide necessary to sponify esters present in 1g of the substance.
Determination of peroxide value: The peroxide value is the number of milli-equivalents of active oxygen that expresses the amount of peroxide contained in 1000g of the substance.
Determine the Reichert Meissel value (R.M value): Reichert Meissel value is the number of milligram of N/10 potassium hydroxide solution required to neutralize the volatile acids obtained by the distillation of 5gm of the fat.
Determination of Iodine value: The iodine value is the number which expresses in grams the quantity of halogen, calculated as iodine, which is observed by 100g of the substance under the desired condition.
Determination of Un-saponification Value: The material is completely saponifiable with alcoholic KOH solution & extracted with petroleum ether. This extract was evaporated and the residue was weighed. Un saponifiable material is this residue- the fatty acid present in it.
Physicochemical Parameters of Cinnamonum zeylanicum
Percentage yield of the crude oil of Cinnamonum zeylanicum
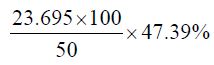
Weight of the empty RD bottle= 19.761
Weight of the RD bottle with oil = 34.456
Relative Density of the oil = 0.96
a) The acid value of Cinnamonum zeylanicum oil comes out to be
Concordant reading (Vs) = 6.8 ml
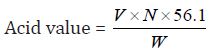
=6.8 x 0.1 x 56.1/ 1
=38.148
b) The Saponification value of Cinnamonum zeylanicum oil comes out to be
Concordant reading (Vs) = 10 ml
Concordant reading (Vb) = 11.5 ml
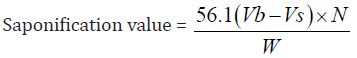
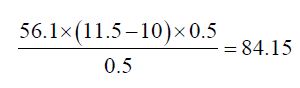
c) The ester value of Cinnamonum zeylanicum oil comes out to be
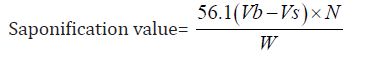
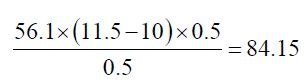
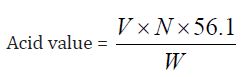
= 6.8×0.1×56.1 1
= 38.148
Ester value = Saponification value – Acid value
= 84.15 - 38.148 = 46.002
d) The Peroxide value of Cinnamonum zeylanicum oil comes out to be
Concordant reading (Vs) = 1.5
Concordant Reading (Vb) = 1.0 ml
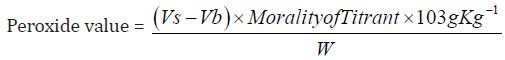
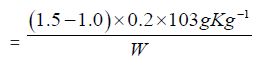
e) The Reichter Meissel value of Cinnamonum zeylanicum oil comes out to be
Concordant reading = (A - B) x N x 11
Reichter Meissel Value
= 3.86 - 2.70 x 0.2 x 11
= 2.55
f) The Iodine value of Cinnamonum zeylanicum oil comes out to be
Concordant Reading (Vb) = 25.7ml
Concordant reading (Vs) = 13.0 ml
Iodine value = (Vb- VS) x 1.269/w
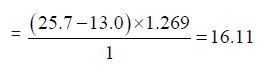
g) Un-Saponification value of oil:
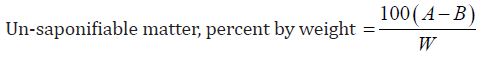
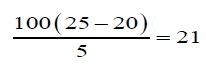
The percentage yield of Cinnamonum zeylanicum was found to be 47.39% and relative density or specific gravity of Cinnamonum zeylanicum oil was found to be 0.96, which indicates that the oil of the plant could be used on commercial scale. The colour of the Cinnamonum zeylanicum oil was yellowish with a characteristic odour. However the oils are liquid at room temperature which may be due to the presence of higher unsaturated molecular fatty acids like oleic acid and linoleic acid and other unsaturated fatty acids.
Peroxide value this measures the deterioration of oil from oxidation. Therefore, the low peroxide value 10.3g/kg obtained from Cinnamonum zeylanicum oil indicates that the oil can be kept for a very long period of time. Initial peroxide values for the edible oils and fat ranged between 0.9 and 15.9 meq/kg-oil. The peroxide value of the plant is within the FAO/WHO and TBS standards for edible vegetable oils. Peroxide values increased during storage. The rate of peroxidation differed from oil to oil as related to different treatment to which the oils were subjected. In general the peroxide value increased with storage time. Oils exposed to both atmospheric oxygen and light showed a much larger increase in peroxide value during storage.
The acid value 38.148 mg KOH/g obtained from the Cinnamonum zeylanicum oil is high. The higher the acid value of oil, the lower is its storage quality and vice-versa, this shows that the Cinnamonum zeylanicum oil have an excellent storage quality.
The saponification value of the Cinnamonum zeylanicum oil was found to be 84.15 mg KOH/g, which means that it has potential for soap production. This indicates that the oil could be used in soap making since its saponification value is high.
The ester value of the Cinnamonum zeylanicum oil was found to be 46.002, being the low ester value of the concerned oil the oil can be kept for long period, so possess high durability which is due to the presence of short range acids.
This is a measure of the proportion of unsaturated acid or fat and oil present, but the test measures the amount of iodine absorbed per gram of sample. The determination of iodine value measures the reaction of the double bonds with halogen. The iodine value of Cinnamonum zeylanicum oil was found to be 16.11g/100g which is low. The oil shows quite degree of unsaturated fatty acid which indicates that the oil is suitable for consumption and can also be used as non-drying oil, which is useful in the manufacture of soap.
The Reichert-Meissel is a value determined when examining fat. The Reichert value is an indicator of how much volatile fatty acid can be extracted from fat through saponification. It is equal to the number of milliliters of 0.1 normal hydroxide solution necessary for the neutralization of the water-soluble volatile fatty acids distilled and filtered from 5 grams of a given saponified fat. The R.M value gives the natural composition of the fat and is used for detection of fat adulteration. Butter that has high percentage of short-chain fatty acids has highest Reichert-Meissel number compared to margarine. The R.M. value of Cinnamonum zeylanicum was found to be 2.55.
The un-saponification value of the Cinnamonum zeylanicum oil was found to be 21mg KOH/g, which means that the concerned oil can be consumed.
The results in this analysis indicated good quality of the Cinnamonum zeylanicum oil. Chemical analysis presented saponification value, peroxide value, acid value, free fatty acid and Iodine values that fell within the range of those acceptable as having good potential for soap production and with an excellent storage property. Before storage, the physicochemical characteristics of the imported edible vegetable oil largely conform to both local standards set by TBS and international standard of the FAO/WHO. The physicochemical properties changed significantly depending on storage time and also with the mode of storage. With storage, the quality of oils deteriorated. The peroxide value of the oils and fat exposed to atmospheric oxygen and light being the highest changing property. Oils kept in tightly sealed containers and stored in the dark exhibited very minor changes in their physicochemical properties.


