Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nagy Abdulsamee*
Received: May 12, 2017 Published: May 23, 2017
Corresponding author: Nagy Abdulsamee, Consultant Prosthodontics, Professor, and Head of Dental Biomaterials, Faculty of Dentistry, Modern University for Technology & Information (MTI), Egypt
DOI: 10.26717/BJSTR.2017.01.000104
The breakthrough for dental laser systems came in the mid 1990’s. Among the various laser types with corresponding wavelengths, Er:YAG laser systems quickly began establishing themselves as compact and versatile additions to the dentist’s repertoire, predominantly for performing hard tissue applications. Research has shown that their wavelengths are ideally suited for both soft and hard tissue procedures due to their high absorption in water and hydroxyl apatite. Therefore Er YAG laser is considered one of the most versatile with regard to the number of possible treatment options, as their wavelength can be effectively used in the field of soft and hard tissue surgery, periodontics, endodontics, implantology, cavity preparation, and tooth whitening. The versatility of the instrument, combined with the latest achievements in Er YAG laser technology, compact design and affordability, should appeal to dental professionals seeking to optimize the procedures they currently perform and expand the number of services they offer.
Keywords: Lasers basics; Laser tissue interaction; Chromophores; Er; YAG laser applications in dentistry
Abbreviations: Nd: Neodymium; NIR: Near Infra Red; FIR: Far Infra Red; SEM: Scanning Electron Microscopy
Lasers have become extremely important either as an adjunct tool or a treatment devices in dental field. They have a variety of applications both in soft and hard dental tissue treatments. It is therefore crucial for the clinician to have an understanding of laser basics. In 1956, Thomas Maiman exposed an extracted natural tooth to his prototype Ruby (694 nm) laser; the nature of the wavelength and target chromophore, together with the laser power resulted in charring of the hard tissue element and transmission of laser energy to the tooth pulp [1]. Following the early clinical experiences of Goldman and others such as Polanyi and Jako in the 1960s, the development of Argon, Neodymium (Nd) YAG and Carbon Dioxide lasers in general areas of surgery led to a gradual introduction of these wavelengths in surgical procedures in the mouth. These early lasers have continuous-wave emission mode, which gave rise to potential for conductive heat damage. This was addressed by the introduction of pulsed-emission lasers, which allowed selective destruction of abnormal or diseased tissue, while leaving surrounding normal tissue undisturbed. The first lasers to fully exploit this principal of ‘selective thermolysis’ were the pulsed dye lasers introduced in the late 1980s.
The possibilities for laser use in dentistry did not occur until 1989, with the production of the American Dental Laser for commercial use. This laser, using an active medium of Nd: YAG [2]. The great advance for dental lasers came in the mid 1990s, with various laser types (Diode laser, Nd: YAG, Er, Cr: YSGG, Er: YAG, CO2) with corresponding wavelengths (810-890 nm, 1064 nm, 2780 nm, 2940 nm, 10600 nm) becoming available to the dentists to address their needs for hard and soft tissue procedures. Soft tissue lasers [near infra red (NIR)] are characterized by a high absorption in chromophores (hemoglobin and melanin) found in soft tissue, resulting in excellent soft tissue incision, ablation and coagulation performance as well as antimicrobial effectiveness, due to relatively deep highly localized tissue heating. Hard tissue lasers [Far infra red (FIR)] (Figure 1) are highly absorbed in (carbonated) hydroxyapatite and water chromophores and are thus able to finely ablate hard tissues without heating of the surrounding tissue (Figures 1 & 2) [3].
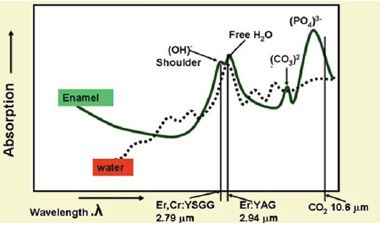
Figure 1: Absorption coefficients of carbonated hydroxyapatite verses laser wavelength. The absorption peaks Represent component radicals of the molecule (hydroxyl, free-water, carbonate, phosphate). The dotted line represents the absorption of laser energy in whole water.
Figure 2: Absorption coefficients of carbonated hydroxyapatite verses laser wavelength. The absorption peaks Represent component radicals of the molecule (hydroxyl, free-water, carbonate, phosphate). The dotted line represents the absorption of laser energy in whole water.
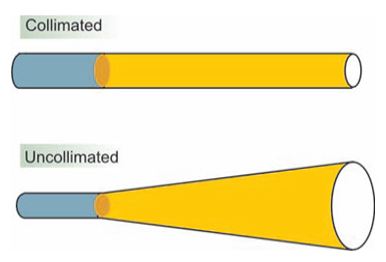
Figure 3: The difference between collimated light (laser) and uncollimated light.

Figure 4: Laser light is monochromatic and coherent.
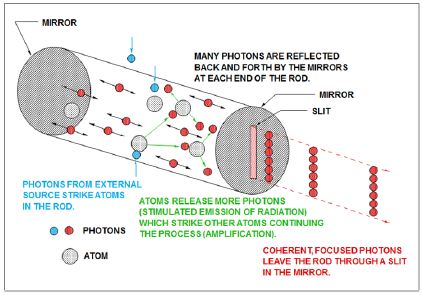
Figure 5: Production of laser
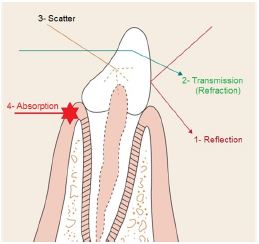
Figure 6: Possible laser light - tissue interactions.
In 1989, experimental work by Keller and Hibst [4] using a pulsed Erbium YAG (2,940 nm) laser, demonstrated its effectiveness in cutting enamel, dentine and bone [4]. This laser became commercially available in 1995 and, shortly followed by a similar Er,Cr:YSGG (erbium chromium: yttrium scandiumgallium garnet - 2,780 nm) laser in 1997, amounted to a laser armamentarium that would address the surgical needs of everyday dental hard tissue treatment (Figure 2).
There are four properties that are common to all laser types:
Beam directionality (collimation) (Figure 3), Monochromaticity, Spatial and temporal coherence of the beam (Figure 4), and High intensity of the beam (Figures 3 & 4) [5]. The intensity, directionality, and monochromaticity of laser light allow the beam to be expanded, or focused quite easily [6].
The amplification of light within the laser cavity sets laser light apart from other sources. For most visible light applications, laser represents a conversion from lamplight to an amplified monochromatic form [7]. The high power possible with lasers (especially peak power) is achieved through resonance in the laser cavity. The scientific principle on which lasers are based is stimulated emission. With spontaneous emission, electrons transition to the lower level in a random process. With stimulated emission, the emission occurs only in the presence of photons of a certain change. The critical point is maintaining a condition where the population of photons in a higher state is larger than that in the lower state. To create this population inversion, a pumping energy must be directed either with electricity, light, or chemical energy.
In clinical dentistry, laser light is used to effect controlled and precise changes in target tissue, through the transfer of electromagnetic energy [8]. Light energy interacts with a target medium (e.g. oral tissue) in one of four ways [9] (Figure 6):
Reflection: When either the density of the medium or angle of incidence are less than the refractive angle, total reflection of the beam will occur. The incident and emergence angles of the laser beam will be the same for true reflection or some scatter may occur if the medium interface is non-homogenous or rough.
Transmission: Laser beam enters the medium and emerges distally without interacting with the medium. The beam exits either unchanged or partially refracted.
Scatter: There is interaction between the laser beam and the medium. This interaction is not intensive enough to cause complete attenuation of the beam. Result of light scattering is a decrease of laser energy with distance, together with a distortion in the beam (rays travel in an uncontrolled direction through the medium).
Absorption: The incident energy of the beam is absorbed by the medium and transferred into another form of energy. Absorption is the most important interaction. Each wavelength has specific chromophores that absorb their energy. This absorbed energy is converted into thermal and and/or mechanical energy that is used to perform the work desired. Near infrared lasers like diodes and Nd: YAGs are mostly absorbed by pigments such as hemoglobin and melanin. Erbium and CO2 lasers are predominantly absorbed by water, with erbium wavelengths also exhibiting some hydroxyapatite absorption [10,11].
Absorption requires an absorber of light, termed chromophores, which have a certain affinity for specific wavelengths of light. The primary chromophores in the intraoral soft tissue are [12]:
1. Melanin, 2. Hemoglobin, 3. Water, 4. and in dental hard tissues (Water & Hydroxyapatite), and 5. Phosensitive materials in visible light cured polymeric materials (Camphoroquinon & α Diketone).
The most important and significant tissue alterations are dependent on the temperature of the tissue after absorption of the laser radiation, as follows:
At 37°C; no measurable effects are observed for the next 5°C above this.
The first mechanism by which tissue is thermally affected can be attributed to conformational changes of molecules. These effects, accompanied by bond destruction and membrane alterations due to hyperthermia at 42-50°C. If such a hyperthermia lasts for several minutes, a significant percentage of the tissue will already undergo necrosis.
At 60°C, denaturation of proteins and collagen occurs which leads to coagulation of tissue and necrosis of cells. The corresponding macroscopic response is the visible paling of the tissue.
At higher temperatures (>80°C), the cell membrane permeability is drastically increased, thereby destroying the otherwise maintained equilibrium of chemical concentrations.
At 100°C, water molecules contained in most tissues start to vaporize. Due to the large increase in volume during this phase transition, gas bubbles are formed inducing mechanical ruptures and thermal decomposition of tissues.
At temperatures exceeding 150°C, carbonization takes place which is observable by the blackening of an adjacent tissue and the escape of smoke (plume).
Finally, melting may occur. The temperature must have reached a few hundred degrees Celsius to melt the tooth substance which mainly consists of hydroxyl apatite crystals (a chemical compound of calcium and phosphate).
There are two distinct wavelengths that use erbium: Erbium, chromium: YSGG (2780 nm) has an active medium of a solid crystal of yttrium scandiumgallium garnet that is doped with erbium and chromium and Erbium: YAG (2940 nm) has an active medium of a solid crystal of yttrium aluminum garnet that is doped with erbium. Caries removal and tooth preparation are easily accomplished by both the lasers. The Er:YAG laser [14,15].
It has a number of advantages. It produces clean, sharp margins in enamel and dentin. In addition, pulpal safety is not a significant concern, because the depth of energy penetration is negligible. When the Er:YAG laser is used for caries removal, the patient usually does not require local anesthesia. The laser is antimicrobial when used within root canals and on root surfaces, and it removes endotoxins from root surfaces. Finally, vibration from the Er:YAG laser is less severe than that from the conventional high-speed drill, and it is less likely to provoke discomfortor pain.
The prime chromophore in current laser application with hard tissue is water; the absorption peak at around 3.0 mm wavelength identifies the Er:YAG and Er,Cr:YSGG wavelengths as the lasers of choice (Figure 2). The first dental laser - the Nd: YAG 1,064 nm - was marketed as being suitable in tooth cavity preparation - a claim that was quickly deemed to be erroneous for clinical relevance. Early research into this claim supported the ablative effect of the 1,064 nm wavelength on accessible pigmented carious lesions, [16,17] but whenever healthy enamel and dentine was exposed to the laser energy, the comparatively long pulse width and associated heat transfer, combined with the lack of water spray resulted in thermal cracking and melting of hydroxyl apatite together with high intrapulpal temperature rise [18-20].
Although there is a high absorption peak of CO2 laser by carbonated hydroxyl apatite, its continuous wave emission of laser energy and lack of axial water coolant results in rapid carbonisation, cracking and melting of tooth tissue. Therefore the carbon dioxide wavelength is impractical for restorative dental procedures [21]. With the Erbium group of lasers the free-running micropulse emission mode results in rapid and expansive vaporization of interstitial water and dissociation of the hydroxyl radical in the hydroxyl apatite crystal causing an explosive dislocation of the gross Structure [22,23].
Clinically, this is seen as ejection of micro-fragments of tooth tissue within the laser plume and the change in pressure in the immediately surrounding air results in an audible “popping” sound. In target tissue that has greater water content (caries > dentine > enamel), the popping sound is louder. With experience, this can aid the clinician in selectively ablating carious verses non-carious tissue. Compared to near infra-red wavelengths, the explosive outward effect of Erbium laser energy results in minimal thermal diffusion through the tooth structure. Co-axial with this laser is a water spray, to aid in dispersing ablation products and to provide cooling of the target site. The development of ultra-short pulse laser emissions of the Erbium group of wavelengths appears promising in reducing the conductive heat potential, whilst increasing the rates of tissue ablation. Nonetheless, both laser wavelengths allow cavity preparation within acceptable clinical parameters [24].
The Er:YAG laser was introduced in 1974 by Zharikov et al. as a solid-state laser that generates a pulsed laser with a wavelength of 2,940 nm. Of all lasers emitting in the near- and mid-infrared spectral range, the absorption of the Er:YAG laser in water is the greatest because its 2,940 nm wavelength (Figure 1) coincides with the large absorption band for water. The absorption coefficient of water of the Er:YAG laser is theoretically 10,000 and 15,000- 20,000 times higher than that of the CO2 and the Nd: YAG lasers, respectively. Since the Er:YAG laser is well absorbed by all biological tissues that contain water molecules, this laser is indicated not only for the treatment of soft tissues but also for ablation of hard tissues. The FDA approved the pulsed Er:YAG laser for hard tissue treatment such as caries removal and cavity preparation in 1997, for soft tissue surgery and sulcular debridement in 1999, and for osseous surgery in 2004 [25-27]. Therefore the affinity of the Er:YAG laser light to living tissue is extremely high and ablation of hard tissue as well as soft tissue is possible. The high absorption by water limits collateral thermal damage to the surrounding tissue. In the case of hard tissue, the amount of water contained within the tissue is small and heat generation is present but can be controlled with water irrigation [28].
Many systems used the irrigation water as the actual target, and eliminate the hard tissue through kinetic energy delivered by the microburst principle whereby the explosive force of vaporization of the thin film of water is transferred to the hard tissue, thereby ablating it. The thickness of the denatured layer of the root cementum and dentin following Er:YAG laser irradiation of the root surface under water irrigation is reported to be 5-15 μm [29-31]. The mechanism behind ablation is firstly through photothermal evaporation where the light energy is absorbed by water in the hard tissue itself and in other organic substances and secondly by the mechanical effect already mentioned, bringing about tissue ablation through the microburst principle, also known as the micro explosion concept where the water vapor pressure build-up created by the extremely violent evaporation of water exceeds the threshold of the tissue. Ablation by micro explosion is referred to as photomechanical ablation or thermo mechanical ablation [32,33].
Calculus is a multi-porous calcified substance, which contains water not only as a constituent of the substance but also within its pores. Hence, in normal biological conditions, calculus is one of the more easily ablated tissues using the Er: YAG. A more recent laser based on an Er, Cr: YSGG medium emits laser light similar to the Er:YAG laser at a wavelength of 2.78 μm, and has been reported to have similar efficacy concerning calculus removal [34].
The Er:YAG laser offers significant advantages over other conventional osteotomy techniques like a noncontact intervention, no mechanical vibration, free and elaborate cut geometries and aseptic effects. The Er:YAG laser is a state of the art and innovative bone cutting technique with a high potential for future applications and trends in oral surgery and implant dentistry [35].
Among all the lasers used in the field of dentistry, the Er:YAG laser has been reported to be the most promising laser for periodontal treatment [36]. Its excellent ability to effectively ablate hard tissues and dental calculus without producing major thermal side-effects to adjacent tissue has been demonstrated in numerous studies [37-40]. Scanning electron microscopy (SEM) observations from recent studies showed that the clinical use of an Er:YAG laser resulted in a smooth root surface morphology, even at higher energy settings [41-43]. Er:YAG lasers have been often discussed as a treatment options for removal of subgingival and periimplant biofilms; available evidence suggests that subgingival and submucosal debridement with Er:YAG laser treatment may reduce periodontal and peri-implant mucosal inflammation [44-46]. Ablation of subgingival biofilms and in particular decontamination of titanium implant surfaces with an Er:YAG laser seem to be a promising approach and warrants further investigations [47].
It has been estimated that roughly 30% to 50% of the US population snore and almost 1/3 suffer from sleep apnea. However, only 5% have been diagnosed and treated [48-49]. Snoring and sleep apnea result from obstructed airways. This can be an outcome of many different factors such as anatomic deviations, tumors, polyps, allergy, large adenoids and tonsils, large uvula or a long soft palate [50-53]. Heavy snoring is sometimes called “heroic” snoring and may affect bed partners, causing severe marital conflicts. There are many benefits of the NightLase® treatment, such as no need for anesthesia, no pain and only three short 20-minute sessions with immediate results. Nightlase uses the photothermal capabilities of Er YAG laser to convert and initiate the formation of new collage in mucosal tissues in the oropharynx, soft palate, and uvula. The heat generated allows the collagen to reform resulting in tightening of the soft palate and surrounding tissues. This caused a rise of the soft palate and tightening the tissues of the oropharynx resulting in an improvement in the airway [54].
Laser etching was preformed with an Er:YAG laser device (Fotona 1210, Ljubljana, Slovenia) [Figure 2] of a wavelength of 2940 nm at 20 HZ, SP mode for 25 s. The two different power settings used in this study were 100 and 150 mj. laser etching at 150 and 100 mj was adequate for bond strength but the failure pattern of brackets bonded with laser etching is dominantly at adhesive - enamel interface and is not safe for enamel during debonding. Laser etched enamel using Er:YAG laser etching at 1 W (100 mJ, 10 Hz) would provide both adequate demineralization prevention and bracket bond strength [55].
In a recent study of caries inhibitory effect of remineralizing agents (Casein Phosphopeptide-Amorphous Calcium Phosphate and Casein Phosphopeptide-Amorphous Calcium Fluoride Phosphate) on human enamel followed with Er:YAG irradiation. It was proven that Er:YAG laser treatment increased resistance of the treated enamel to acid dissolution [56].
Based on the results of a study compared laser vs bur for bone cutting in impacted mandibular third molar surgery, they concluded that possibility of bone cutting using lasers is pursued. The osteotomy is easily performed with laser and the technique is minimally invasive surgical procedures. The use of Er:YAG laser may be considered as an alternative tool to surgical bur specially in anxious patients [57].
Laser irradiation makes structural and chemical changes on the dental hard tissues. These changes alter the level of solubility and permeability of dentin. Consequently, the bond strength of adhesive systems on dentine surfaces may be affected in clinical practice. The Er:YAG laser is safe for cavity preparation in primary teeth [58].
Cavity preparation with an Er:YAG laser could be considered as an alternative to the conventional method of drilling [59]. Some authors showed that there is no statistically significant difference could be observed in the fracture strength of dentin beams when treating them either with Er:YAG and Er, Cr: YSGG laser irradiation or mechanically by a fine diamond bur in a high speed hand piece. Additionally, no statistically significant difference could be observed between treated and untreated specimens [60]. Er:YAG laser is more comfortable and pleasant for the patient, compared to conventional drill. Also it reduces tooth hypersensivity and microbial load within the cav¬ity [61]. Ablated dentin with different parameters of Er:YAG laser energy with powers below 3 W make no cracks. These facts are adjunct to suitable dentin surface treatment by Er:YAG laser, making Er:YAG laser a desirable alternative method for cavity preparation [62]. Ablation of dental hard tissues was achieved using the Er:YAG laser operating at high pulse repetition rates with minimal peripheral thermal damage [63].
In addition, since water is the primary absorber of Er:YAG radiation and demineralized areas are more porous and have a higher water content, the ablation rate is significantly higher for demineralized enamel [64] and dentin vs sound tissues. The Er:YAG laser may be used in conservative dentistry as an alternative to conventional instruments and in association with orthophosphoric acid, with several advantages, such better strength bond [65], reduced micro leakage [66], and also lower discomfort and higher patient satisfaction [67]. In an in vitro study, even if considered as preliminary due to the limited number of samples, it is confirmed that Er:YAG can be employed also in dental traumatology, to restore frontal teeth after coronal fracture, with the advantage of improved adhesion of the dental fragment to the tooth, in particular by decreasing micro leakage [68].
Irradiation of Nd: YAG, Er: YAG, CO2, Tm: Yap, diode or ytterbium fiber lasers may be considered as an efficient way to reduce shear bond strength of ceramic bracket and debonding time. This technique is a safe way for removing ceramic brackets while the intrapulpal temperature and enamel surface were minimally affected, along with reduced ceramic bracket failure [69]. The Er-YAG laser emits at 2904 nm, which corresponds to the main absorption peak of water [4]. Therefore, an Er- YAG laser may be highly absorbed by the adhesive bond¬ing resin containing water or residual monomer [70].
Advantages of ytterbium fiber laser are high optical quality, compact size, extended lifetime and flexible mode of operation. Thus, it was selected for ceramic bracket removal [71]. Er-YAG laseraided debonding, with or without water-cooling, was effective for debonding ceramic brackets by reducing resin shear bond strength. Er-YAG laser application with water-cooling appeared to be a safer option by reducing resin shear bond strength and reducing the likelihood of intrapulpal temperature increase while debonding ceramic brackets [70].
Some authors concluded that lasers may be used in soft tissue surgery of the oral cavity, as long as the biological effects related to the use of each type of laser are understood and respected. The Er:YAG laser may be the laser of choice for biopsies of the oral mucosa because of the minimum histological artefacts observed, ensuring a valid histological evaluation, followed by the CO2 laser at 3.5W in pulsed mode, es-pecially when the surgeon needs more hemostasis on the surgical field [72] .
Both, Er:YAG and CO2 lasers may be effective in the treatment of benign neoplastic and tumorous lesions of the oral soft tissues being an alternative to conventional surgery. They shorten postoperative healing time, eliminate or soothe inflammation, reduce perisurgical pain. Thanks to effective hemostasis, CO2 laser may be used to remove richly vascularized lesions and in compromised patients. Healing neither changes the tissue profile nor causes its loss, ensures fast and good adaptation of the changes. A major limitation of the laser technique application is thermal injury to the surrounding tissues, charring and melting of wound margins with CO2 laser, especially during multiple passage of the beam and with excessive power for a small lesion. This may promote tissue defragmentation and thus frequently makes histopathological evaluation impossible. CO2 laser is contraindicated for lesions smaller than 3 mm in diameter as they may get defragmented making histopathological evaluation impossible. Therefore, for the majority of minor oral surgeries Er:YAG laser is a better choice [73].
Based on the experience performed on 5 years of Special Care patients conservative treatments we may affirm the Er:YAG laser may be considered as a good way to improve the cooperation, to reduce anxiety related to rotating instruments and to reach better results with equal or shorter operating times [74].
Based on the limited number of investigations, at present, only one particular wavelength appears to be able to perform direct photo bleaching (or photooxidation), that is, KTP (532 nm). When KTP is used in combination with a bleaching gel containing a chromophore (sulphorhodamine) allowing the absorption of the laser light, photodynamic reactions can be induced (photochemical activation of the gel with limited photothermal activation). This combination of wavelength and specifically dyed bleaching gel also allows for safe bleaching (no damage of the enamel, no heating of the pulp) when the guidelines of the manufacturer are followed.
At present a number of wavelengths are not recommended for laser bleaching: Nd: YAG, Er: YAG, and CO2. Combination devices consisting of LED-diode laser do not result in enhanced lightening and are in fact not effective. When using high power diode lasers for bleaching care has to be taken so as not to overheat the pulp. Also diode lasers are not really advocated for laser bleaching except when the wavelength is used in combination with a bleaching gel containing wavelength specific absorbers [75].
Caries Prevention: Resistance of the tooth surface to penetration of cariogenic agents plays an important role in prevention of caries. Er YAG laser can be successfully used to increase resistance of a newly erupted permanent tooth in children and adolescents to acid erosion [76,77]. Er:YAG laser cavity preparation allows minimally invasive treatment of dental caries and also shows excellent acceptance among both young children and their parents. The choice of optimal energy parameters is a requirement for successful laser caries treatment in pediatric dentistry [78].
Restoration, Pit and Fissure Sealants: Laser can also be used for tooth surface preparation prior to the application of pit and fissure sealants. Laser can be applied for conditioning, cleaning and disinfection of pits and fissures as well [79]. Er YAG laser at lower wavelengths causes only macro-roughening of pits and fissures [80].
Er YAG in Endodontics: Application of Er:YAG laser for pulp coagulation has also shown more favorable results after 2 years in comparison with calcium hydroxide [81,82].
Er YAG in Soft Tissue Applications of Laser: Er:YAG laser can be used for frenectomy in infants with tight maxillary frenumor for upper and lower frenecto¬my in infants with severe ankyloglossia [83] .
Traumatology: Er:YAG laser can be used for fusion and sealing of dentinal tubules in case of fractured teeth or open dentinal tubules. By doing so, the permea¬bility of tubules and the consequent tooth hypersensitivity will decrease [84].
Exposure of Unerupted Teeth for Orthodontic Purposes: For soft tissue removal and exposure of unerupted teeth for orthodontic purposes, Er, YAG is used [85].
The application of sandblasting technique accompanied by Er:YAG laser irradiation to an amalgam filling in a tooth can provide suitable surface for bonding of orthodontic brackets to that amalgam [86].
The basics of laser science, tissue effects of dental lasers, Er YAG laser wave length and their chromophores, and some important applications of this laser in dentistry have been discussed. It is important for the clinician to understand these principles to take full advantage of the features of Er YAG laser and provide safe and effective treatment.


